当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptide/Cas9 nanostructures for ribonucleoprotein cell membrane transport and gene edition
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7sc03918b Irene Lostalé-Seijo 1, 2, 3, 4, 5 , Iria Louzao 1, 2, 3, 4, 5 , Marisa Juanes 1, 2, 3, 4, 5 , Javier Montenegro 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1039/c7sc03918b Irene Lostalé-Seijo 1, 2, 3, 4, 5 , Iria Louzao 1, 2, 3, 4, 5 , Marisa Juanes 1, 2, 3, 4, 5 , Javier Montenegro 1, 2, 3, 4, 5
Affiliation
|
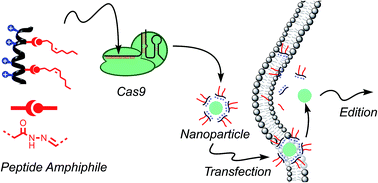
The discovery of RNA guided endonucleases has emerged as one of the most important tools for gene edition and biotechnology. The selectivity and simplicity of the CRISPR/Cas9 strategy allows the straightforward targeting and editing of particular loci in the cell genome without the requirement of protein engineering. However, the transfection of plasmids encoding the Cas9 and the guide RNA could lead to undesired permanent recombination and immunogenic responses. Therefore, the direct delivery of transient Cas9 ribonucleoprotein constitutes an advantageous strategy for gene edition and other potential therapeutic applications of the CRISPR/Cas9 system. The covalent fusion of Cas9 with penetrating peptides requires multiple incubation steps with the target cells to achieve efficient levels of gene edition. These and other recent reports suggested that covalent conjugation of the anionic Cas9 ribonucleoprotein to cationic peptides would be associated with a hindered nuclease activity due to undesired electrostatic interactions. We here report a supramolecular strategy for the direct delivery of Cas9 by an amphiphilic penetrating peptide that was prepared by a hydrazone bond formation between a cationic peptide scaffold and a hydrophobic aldehyde tail. The peptide/protein non-covalent nanoparticles performed with similar efficiency and less toxicity than one of the best methods described to date. To the best of our knowledge this report constitutes the first supramolecular strategy for the direct delivery of Cas9 using a penetrating peptide vehicle. The results reported here confirmed that peptide amphiphilic vectors can deliver Cas9 in a single incubation step, with good efficiency and low toxicity. This work will encourage the search and development of conceptually new synthetic systems for transitory endonucleases direct delivery.
中文翻译:

核糖核蛋白细胞膜转运的肽/ Cas9纳米结构和基因编辑
RNA指导的核酸内切酶的发现已成为基因编辑和生物技术最重要的工具之一。CRISPR / Cas9策略的选择性和简单性允许直接靶向和编辑细胞基因组中的特定基因座,而无需蛋白质工程。但是,转染编码Cas9和指导RNA的质粒可能会导致不良的永久重组和免疫原性反应。因此,瞬时Cas9核糖核蛋白的直接递送构成CRISPR / Cas9系统的基因编辑和其他潜在治疗应用的有利策略。Cas9与穿透肽的共价融合需要与靶细胞进行多个温育步骤,以实现有效水平的基因编辑。这些和其他最近的报道表明,由于不希望的静电相互作用,阴离子Cas9核糖核蛋白与阳离子肽的共价缀合将与核酸酶活性受阻有关。我们在这里报告了由两亲性穿透肽直接递送Cas9的超分子策略,该两亲性穿透肽是通过在阳离子肽支架和疏水性醛尾之间形成键而制备的。与迄今为止描述的最佳方法之一相比,肽/蛋白质非共价纳米颗粒具有相似的效率和更低的毒性。据我们所知,本报告构成了第一个使用穿透性肽载体直接递送Cas9的超分子策略。此处报道的结果证实,肽两亲性载体可在单个孵育步骤中递送Cas9,效率高且毒性低。这项工作将鼓励搜索和开发用于瞬时内切核酸酶直接递送的概念上新的合成系统。
更新日期:2017-11-02
中文翻译:

核糖核蛋白细胞膜转运的肽/ Cas9纳米结构和基因编辑
RNA指导的核酸内切酶的发现已成为基因编辑和生物技术最重要的工具之一。CRISPR / Cas9策略的选择性和简单性允许直接靶向和编辑细胞基因组中的特定基因座,而无需蛋白质工程。但是,转染编码Cas9和指导RNA的质粒可能会导致不良的永久重组和免疫原性反应。因此,瞬时Cas9核糖核蛋白的直接递送构成CRISPR / Cas9系统的基因编辑和其他潜在治疗应用的有利策略。Cas9与穿透肽的共价融合需要与靶细胞进行多个温育步骤,以实现有效水平的基因编辑。这些和其他最近的报道表明,由于不希望的静电相互作用,阴离子Cas9核糖核蛋白与阳离子肽的共价缀合将与核酸酶活性受阻有关。我们在这里报告了由两亲性穿透肽直接递送Cas9的超分子策略,该两亲性穿透肽是通过在阳离子肽支架和疏水性醛尾之间形成键而制备的。与迄今为止描述的最佳方法之一相比,肽/蛋白质非共价纳米颗粒具有相似的效率和更低的毒性。据我们所知,本报告构成了第一个使用穿透性肽载体直接递送Cas9的超分子策略。此处报道的结果证实,肽两亲性载体可在单个孵育步骤中递送Cas9,效率高且毒性低。这项工作将鼓励搜索和开发用于瞬时内切核酸酶直接递送的概念上新的合成系统。









































 京公网安备 11010802027423号
京公网安备 11010802027423号