当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A one-pot, copper-catalyzed azidation/click reaction of aryl and heteroaryl bromides in an environmentally friendly deep eutectic solvent
Tetrahedron ( IF 2.1 ) Pub Date : 2017-10-31 , DOI: 10.1016/j.tet.2017.10.050 Arjun Kafle , Scott T. Handy
中文翻译:

在环境友好的深共熔溶剂中进行一锅铜催化的芳基和杂芳基溴化物的叠氮化/点击反应
更新日期:2017-10-31
Tetrahedron ( IF 2.1 ) Pub Date : 2017-10-31 , DOI: 10.1016/j.tet.2017.10.050 Arjun Kafle , Scott T. Handy
|
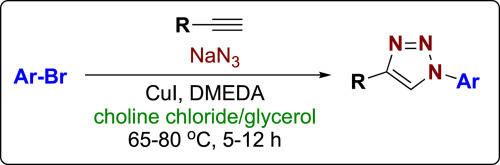
In an effort to avoid the hazards of isolating and handling azides in click chemistry, many groups have turned to in situ generation of azides from halide precursors. This option is readily accomplished for alkyl azides, but is more challenging for aryl azides. In this paper, we discuss our success in transforming aryl bromides into 1,4-disubstituted triazoles employing DMEDA as a ligand with a copper catalyst in a deep eutectic solvent. Further, we are able to recycle the solvent, catalyst, and ligand several times, thereby increasing the attractiveness of this method.
中文翻译:

在环境友好的深共熔溶剂中进行一锅铜催化的芳基和杂芳基溴化物的叠氮化/点击反应
为了避免在点击化学中分离和处理叠氮化物的危险,许多小组已经转向从卤化物前体原位生成叠氮化物。对于烷基叠氮化物,该选择容易实现,但对于芳基叠氮化物则更具挑战性。在本文中,我们讨论了在深共熔溶剂中使用DMEDA作为配体与铜催化剂将芳基溴化物转化为1,4-二取代的三唑的成功方法。此外,我们能够多次循环使用溶剂,催化剂和配体,从而提高了该方法的吸引力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号