当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective annulation of propargyl alcohols with ambident-enols: A Ca(II)-catalyzed trisubstituted benzochromene synthesis
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.tetlet.2017.10.077 Srinivasarao Yaragorla , Abhishek Pareek , Ravikrishna Dada
中文翻译:

炔丙醇与环境型烯醇的区域选择性环化:Ca(II)催化的三取代苯并二甲基苯并戊烯的合成
更新日期:2017-11-01
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.tetlet.2017.10.077 Srinivasarao Yaragorla , Abhishek Pareek , Ravikrishna Dada
|
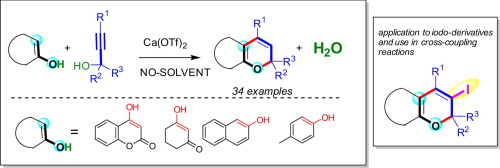
Highly regioselective, 6-endo cyclization between propargyl alcohols and ambident enols such as naphthols, 4-hydroxy coumarin, cyclohexane-1,3-dione, 5,5-dimethylcyclohexane-1,3-dione is described using Ca(OTf)2 under solvent free conditions. The reaction proceeds through a cascade annulation which involves an etherification, Claisen type rearrangement, allene formation and endocyclization. Further, we extended this method to the synthesis of iodo-derivative and demonstrated the reactivity in cross-coupling reactions.
中文翻译:

炔丙醇与环境型烯醇的区域选择性环化:Ca(II)催化的三取代苯并二甲基苯并戊烯的合成
使用Ca(OTf)2在以下条件下描述了炔丙醇与环境烯醇(例如萘酚,4-羟基香豆素,环己烷-1,3-二酮,5,5-二甲基环己烷-1,3-二酮)之间的高度区域选择性,6-内环化。无溶剂条件。反应通过级联反应进行,该级联反应涉及醚化,克莱森(Claisen)型重排,丙二烯形成和内环化。此外,我们将该方法扩展到碘衍生物的合成,并证明了交叉偶联反应的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号