当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Enantioselective Michael–Michael–Michael–Aldol Condensation Reactions: Control of Six Stereocenters in a Quadruple-Cascade Asymmetric Synthesis of Polysubstituted Spirocyclic Oxindoles
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.orglett.7b02962 Prakash D. Chaudhari,Bor-Cherng Hong,Gene-Hsiang Lee
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.orglett.7b02962 Prakash D. Chaudhari,Bor-Cherng Hong,Gene-Hsiang Lee
|
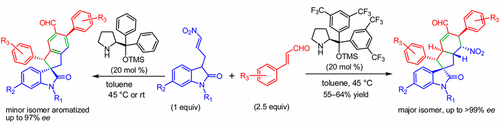
An organocatalyzed enantioselective Michael–Michael–Michael–aldol cascade reaction for the construction of cyclopentane fused spirooxindoles was achieved. The domino reaction provided the spirooxindoles with six contiguous stereocenters including a quaternary center in good yields (55–64%) with excellent enantioselectivities (up to >99% ee). Enantioselective Michael–Michael–Michael–aldol condensation–aromatization reactions of an isomeric product were observed.
中文翻译:

有机催化对映选择性Michael–Michael–Michael–Aldol缩合反应:四级联多不对称合成多取代螺环氧化吲哚中的六个立体中心的控制。
实现了有机催化的对映体选择性迈克尔-迈克尔-迈克尔-奥尔多级联反应,用于构建环戊烷稠合的螺氧并吲哚。多米诺骨牌反应为螺硫醇提供了六个连续的立体中心,包括一个具有良好对映选择性(高达> 99%ee)的高产(55-64%)的四元中心。观察到异构产物的对映选择性迈克尔-迈克尔-迈克尔-醛醇缩合-芳香化反应。
更新日期:2017-11-01
中文翻译:

有机催化对映选择性Michael–Michael–Michael–Aldol缩合反应:四级联多不对称合成多取代螺环氧化吲哚中的六个立体中心的控制。
实现了有机催化的对映体选择性迈克尔-迈克尔-迈克尔-奥尔多级联反应,用于构建环戊烷稠合的螺氧并吲哚。多米诺骨牌反应为螺硫醇提供了六个连续的立体中心,包括一个具有良好对映选择性(高达> 99%ee)的高产(55-64%)的四元中心。观察到异构产物的对映选择性迈克尔-迈克尔-迈克尔-醛醇缩合-芳香化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号