当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Base-Promoted Synthesis of Quinoline-4(1H)-thiones from o-Alkynylanilines and Aroyl Isothiocyanates
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.orglett.7b02993 Anju Modi 1 , Prasenjit Sau 1 , Bhisma K. Patel 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.orglett.7b02993 Anju Modi 1 , Prasenjit Sau 1 , Bhisma K. Patel 1
Affiliation
|
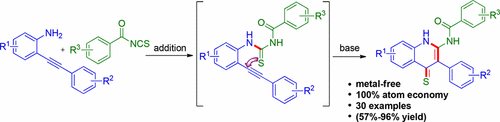
A base-promoted synthesis of quinoline-4(1H)-thiones has been accomplished from the in situ generated o-alkynylthiourea, obtained by reacting o-alkynylanilines with aroyl/acyl isothiocyanates. A 6-exo-digS-cyclization of the in situ generated thiourea is followed by a rearrangement to give quinoline-4(1H)-thiones.
中文翻译:

由邻烷基炔苯胺和芳基异硫氰酸酯碱促合成喹啉-4(1 H)-硫酮
由原位生成的邻炔基硫脲的碱促合成喹啉-4(1 H)-硫酮是通过使邻炔基苯胺与芳酰基/酰基异硫氰酸酯反应获得的。原位产生的硫脲的6- exo - dig S-环化反应,然后进行重排,得到喹啉-4(1 H)-硫酮。
更新日期:2017-11-01
中文翻译:

由邻烷基炔苯胺和芳基异硫氰酸酯碱促合成喹啉-4(1 H)-硫酮
由原位生成的邻炔基硫脲的碱促合成喹啉-4(1 H)-硫酮是通过使邻炔基苯胺与芳酰基/酰基异硫氰酸酯反应获得的。原位产生的硫脲的6- exo - dig S-环化反应,然后进行重排,得到喹啉-4(1 H)-硫酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号