当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hidden complexity of synergistic roles of Dopa and lysine for strong wet adhesion
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7qm00402h Ying Li 1, 2, 3, 4, 5 , Chao Liang 6, 7, 8, 9, 10 , Ling Gao 11, 12, 13 , Shiyu Li 13, 14, 15, 16 , Yizhe Zhang 13, 14, 15, 16 , Jiang Zhang 6, 7, 8, 9, 10 , Yi Cao 6, 7, 8, 9, 10
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7qm00402h Ying Li 1, 2, 3, 4, 5 , Chao Liang 6, 7, 8, 9, 10 , Ling Gao 11, 12, 13 , Shiyu Li 13, 14, 15, 16 , Yizhe Zhang 13, 14, 15, 16 , Jiang Zhang 6, 7, 8, 9, 10 , Yi Cao 6, 7, 8, 9, 10
Affiliation
|
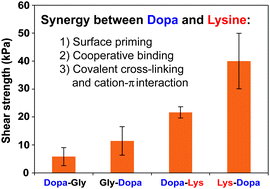
Dopa and lysine are widely found in mussel foot proteins and are suggested to play synergistic roles in wet adhesion; yet, the detailed molecular mechanism remains unclear. Here, using PEG conjugated dipeptides as the model system, we found that the neighboring lysine can significantly enhance surface binding of Dopa through three distinct mechanisms: (1) displacing surface water and ions to increase the effective binding sites; (2) being directly involved in cooperative surface binding in a sequence dependent manner; (3) enhancing cohesion by Michael addition to oxidized species or forming cation–π interactions. This study may be helpful for rational design of biomimetic strong adhesives for biomedical applications.
中文翻译:

多巴和赖氨酸协同作用对强湿粘附力的隐藏复杂性
多巴和赖氨酸广泛存在于贻贝足蛋白中,并被认为在湿粘附中起协同作用。然而,具体的分子机制仍不清楚。在这里,我们使用PEG共轭二肽作为模型系统,我们发现相邻的赖氨酸可以通过三种不同的机制显着增强Dopa的表面结合力:(1)置换表面水和离子以增加有效结合位点;(2)以序列依赖性方式直接参与协同表面结合;(3)通过添加迈克尔到氧化物种或形成阳离子-π相互作用来增强内聚力。这项研究可能有助于合理设计生物医学应用的仿生强力粘合剂。
更新日期:2017-11-01
中文翻译:

多巴和赖氨酸协同作用对强湿粘附力的隐藏复杂性
多巴和赖氨酸广泛存在于贻贝足蛋白中,并被认为在湿粘附中起协同作用。然而,具体的分子机制仍不清楚。在这里,我们使用PEG共轭二肽作为模型系统,我们发现相邻的赖氨酸可以通过三种不同的机制显着增强Dopa的表面结合力:(1)置换表面水和离子以增加有效结合位点;(2)以序列依赖性方式直接参与协同表面结合;(3)通过添加迈克尔到氧化物种或形成阳离子-π相互作用来增强内聚力。这项研究可能有助于合理设计生物医学应用的仿生强力粘合剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号