当前位置:
X-MOL 学术
›
Integr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of amino acid mutations on intra-dimer tubulin–tubulin binding strength of microtubules
Integrative Biology ( IF 1.5 ) Pub Date : 2017-11-08 , DOI: 10.1039/c7ib00113d Ning Liu 1, 2, 3, 4 , Ramana Pidaparti 1, 2, 3, 4 , Xianqiao Wang 1, 2, 3, 4
Integrative Biology ( IF 1.5 ) Pub Date : 2017-11-08 , DOI: 10.1039/c7ib00113d Ning Liu 1, 2, 3, 4 , Ramana Pidaparti 1, 2, 3, 4 , Xianqiao Wang 1, 2, 3, 4
Affiliation
|
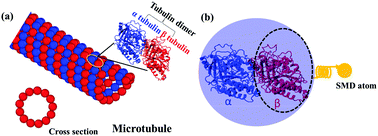
Energetic interactions inside aβ-tubulin dimers of a microtubule (MT) with atomic resolutions are of importance in determining the mechanical properties and structural stability of the MT as well as designing self-assembled functional structures from it. Here, we carry out several comprehensive atomistic simulations to investigate the interaction properties within aβ-tubulin dimers and effect of residue mutations on the intra-dimer tubulin–tubulin (IDTT) binding strength. Results indicate that the force-displacement responses of the dimer could be roughly divided into three stages involving increasing, decreasing, and fluctuating forces. Energetic analysis shows that electrostatic interactions dominate the IDTT binding strength. Further per-residue energetic analysis shows that the major part of the interface interaction energy (approximately 72% for a-tubulin and 62% for β-tubulin) comes from amino acid residues with net charges, namely arginine (ARG), lysine (LYS), glutamic acid (GLU), aspartic acid (ASP). Residue mutations are completed for ARG105 on a-tubulin and ASP251 on β-tubulin to study the effect of mutations on the IDTT binding strength. Results indicate that stiffness, rupture force, and interface interaction energy of aβ-tubulin dimer can be improved by up to 28%, 13% and 28%, respectively. Overall, our results provide a thorough atomistic understanding of the IDTT binding strength within aβ-tubulin heterodimers and help pave the way for eventually designing and controlling the self-assembled functional structures from MTs.
中文翻译:

氨基酸突变对微管内二聚体微管蛋白与微管蛋白结合强度的影响
具有原子分辨率的微管(MT)的α-微管蛋白二聚体内部的能量相互作用对于确定MT的机械性能和结构稳定性以及从中设计自组装功能结构非常重要。在这里,我们进行了几个全面的原子模拟,以研究aβ-微管蛋白二聚体之间的相互作用特性以及残基突变对二聚体微管蛋白-微管蛋白(IDTT)结合强度的影响。结果表明,二聚体的力-位移响应可以大致分为三个阶段,包括增加,减小和波动。能量分析表明,静电相互作用主导了IDTT的结合强度。进一步的每个残基的能量分析表明,界面相互作用能的主要部分(α-微管蛋白约为72%,β-微管蛋白约为62%)来自带有净电荷的氨基酸残基,即精氨酸(ARG),赖氨酸(LYS) ),谷氨酸(GLU),天冬氨酸(ASP)。研究了α-微管蛋白上ARG105和β-微管蛋白上ASP251的残基突变,以研究突变对IDTT结合强度的影响。结果表明,α-微管蛋白二聚体的刚度,断裂力和界面相互作用能分别提高了28%,13%和28%。总体而言,我们的结果提供了对aβ-微管蛋白异二聚体中IDTT结合强度的透彻原子了解,并为最终设计和控制MT自组装功能结构铺平了道路。
更新日期:2017-11-08
中文翻译:

氨基酸突变对微管内二聚体微管蛋白与微管蛋白结合强度的影响
具有原子分辨率的微管(MT)的α-微管蛋白二聚体内部的能量相互作用对于确定MT的机械性能和结构稳定性以及从中设计自组装功能结构非常重要。在这里,我们进行了几个全面的原子模拟,以研究aβ-微管蛋白二聚体之间的相互作用特性以及残基突变对二聚体微管蛋白-微管蛋白(IDTT)结合强度的影响。结果表明,二聚体的力-位移响应可以大致分为三个阶段,包括增加,减小和波动。能量分析表明,静电相互作用主导了IDTT的结合强度。进一步的每个残基的能量分析表明,界面相互作用能的主要部分(α-微管蛋白约为72%,β-微管蛋白约为62%)来自带有净电荷的氨基酸残基,即精氨酸(ARG),赖氨酸(LYS) ),谷氨酸(GLU),天冬氨酸(ASP)。研究了α-微管蛋白上ARG105和β-微管蛋白上ASP251的残基突变,以研究突变对IDTT结合强度的影响。结果表明,α-微管蛋白二聚体的刚度,断裂力和界面相互作用能分别提高了28%,13%和28%。总体而言,我们的结果提供了对aβ-微管蛋白异二聚体中IDTT结合强度的透彻原子了解,并为最终设计和控制MT自组装功能结构铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号