当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Oxidation of Alkynes into 1,2‐Diketone Derivatives by Using a PdII/Lewis‐Acid Catalyst
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-29 , DOI: 10.1002/ajoc.201700556 Jing-Wen Xue 1 , Miao Zeng 1 , Xianfei Hou 1 , Zhuqi Chen 1 , Guochuan Yin 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-29 , DOI: 10.1002/ajoc.201700556 Jing-Wen Xue 1 , Miao Zeng 1 , Xianfei Hou 1 , Zhuqi Chen 1 , Guochuan Yin 1
Affiliation
|
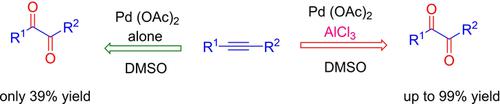
A new synthetic method has been developed for the efficient oxidation of alkynes into 1,2‐diketones by using a PdII/Lewis acid catalytic system using DMSO as the oxidant and solvent. Under our optimized reaction conditions, this approach tolerated a variety of functional groups and afforded 1,2‐diketone derivatives in 62–99 % yields. This work demonstrated that the addition of non‐redox‐active metal ions, such as AlIII, to Pd(OAc)2 could dramatically improve its catalytic efficiency for alkyne oxidation, whilst reactions with Pd(OAc)2 alone were sluggish and afforded a low yield of the corresponding diketone. Based on NMR and UV/Vis analysis, a heterobimetallic PdII/AlIII species was proposed for alkyne oxidation by adding AlCl3 to Pd(OAc)2 in DMSO. The catalytic efficiency of the PdII species was improved by the linkage of AlIII to the PdII species, which made the PdII species more electron deficient. This method may have the potential to become a simple, efficient procedure for the synthesis of 1,2‐diketone derivatives.
中文翻译:

使用PdII /刘易斯酸催化剂将炔烃催化氧化为1,2-二酮衍生物
通过使用DMSO作为氧化剂和溶剂的Pd II /路易斯酸催化体系,已开发出一种新的合成方法,用于将炔烃有效氧化为1,2-二酮。在我们优化的反应条件下,这种方法可以耐受各种官能团,并以62-99%的产率提供1,2-二酮衍生物。这项工作表明,向Pd(OAc)2中添加非氧化还原活性金属离子(例如Al III)可以显着提高其对炔烃氧化的催化效率,而仅与Pd(OAc)2的反应反应缓慢,并提供了相应二酮的收率低。基于NMR和UV / Vis分析,异双金属Pd II / Al III通过在DMSO中将AlCl 3添加到Pd(OAc)2中,提出了一种用于炔烃氧化的物种。Al III与Pd II物种的连接提高了Pd II物种的催化效率,这使Pd II物种更加缺乏电子。该方法可能成为合成1,2-二酮衍生物的简单,有效的方法。
更新日期:2017-11-29
中文翻译:

使用PdII /刘易斯酸催化剂将炔烃催化氧化为1,2-二酮衍生物
通过使用DMSO作为氧化剂和溶剂的Pd II /路易斯酸催化体系,已开发出一种新的合成方法,用于将炔烃有效氧化为1,2-二酮。在我们优化的反应条件下,这种方法可以耐受各种官能团,并以62-99%的产率提供1,2-二酮衍生物。这项工作表明,向Pd(OAc)2中添加非氧化还原活性金属离子(例如Al III)可以显着提高其对炔烃氧化的催化效率,而仅与Pd(OAc)2的反应反应缓慢,并提供了相应二酮的收率低。基于NMR和UV / Vis分析,异双金属Pd II / Al III通过在DMSO中将AlCl 3添加到Pd(OAc)2中,提出了一种用于炔烃氧化的物种。Al III与Pd II物种的连接提高了Pd II物种的催化效率,这使Pd II物种更加缺乏电子。该方法可能成为合成1,2-二酮衍生物的简单,有效的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号