当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Star Polymers Reduce Islet Amyloid Polypeptide Toxicity via Accelerated Amyloid Aggregation
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.biomac.7b01301 Emily H. Pilkington 1 , May Lai 1 , Xinwei Ge 2 , William J. Stanley 3, 4 , Bo Wang 2 , Miaoyi Wang 1 , Aleksandr Kakinen 1 , Marc-Antonie Sani 5 , Michael R. Whittaker 1 , Esteban N. Gurzov 3, 4 , Feng Ding 2 , John F. Quinn 1 , Thomas P. Davis 1, 6 , Pu Chun Ke 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.biomac.7b01301 Emily H. Pilkington 1 , May Lai 1 , Xinwei Ge 2 , William J. Stanley 3, 4 , Bo Wang 2 , Miaoyi Wang 1 , Aleksandr Kakinen 1 , Marc-Antonie Sani 5 , Michael R. Whittaker 1 , Esteban N. Gurzov 3, 4 , Feng Ding 2 , John F. Quinn 1 , Thomas P. Davis 1, 6 , Pu Chun Ke 1
Affiliation
|
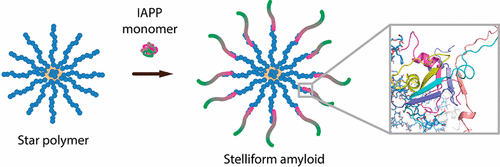
Protein aggregation into amyloid fibrils is a ubiquitous phenomenon across the spectrum of neurodegenerative disorders and type 2 diabetes. A common strategy against amyloidogenesis is to minimize the populations of toxic oligomers and protofibrils by inhibiting protein aggregation with small molecules or nanoparticles. However, melanin synthesis in nature is realized by accelerated protein fibrillation to circumvent accumulation of toxic intermediates. Accordingly, we designed and demonstrated the use of star-shaped poly(2-hydroxyethyl acrylate) (PHEA) nanostructures for promoting aggregation while ameliorating the toxicity of human islet amyloid polypeptide (IAPP), the peptide involved in glycemic control and the pathology of type 2 diabetes. The binding of PHEA elevated the β-sheet content in IAPP aggregates while rendering a new morphology of “stelliform” amyloids originating from the polymers. Atomistic molecular dynamics simulations revealed that the PHEA arms served as rodlike scaffolds for IAPP binding and subsequently accelerated IAPP aggregation by increased local peptide concentration. The tertiary structure of the star nanoparticles was found to be essential for driving the specific interactions required to impel the accelerated IAPP aggregation. This study sheds new light on the structure–toxicity relationship of IAPP and points to the potential of exploiting star polymers as a new class of therapeutic agents against amyloidogenesis.
中文翻译:

星状聚合物通过加速淀粉样蛋白聚集降低胰岛淀粉样蛋白多肽的毒性
在神经退行性疾病和2型糖尿病的整个谱中,蛋白质聚集到淀粉样蛋白原纤维中是普遍存在的现象。对抗淀粉样蛋白生成的常见策略是通过抑制小分子或纳米颗粒的蛋白质聚集来最大程度减少有毒寡聚物和原纤维的数量。然而,自然界中黑色素的合成是通过加速蛋白原纤化来规避有毒中间体的积累而实现的。因此,我们设计并证明了使用星形聚丙烯酸2-羟乙酯(PHEA)纳米结构促进聚集,同时改善人胰岛淀粉样多肽(IAPP)的毒性,参与血糖控制的肽和2型糖尿病的发病机理2糖尿病。PHEA的结合提高了IAPP聚集体中β-折叠的含量,同时呈现了源自聚合物的“固态”淀粉样蛋白的新形态。原子分子动力学模拟显示,PHEA臂可作为棒状支架用于IAPP结合,并随后通过增加局部肽浓度来加速IAPP聚集。发现星形纳米颗粒的三级结构对于驱动促进加速的IAPP聚集所需的特定相互作用至关重要。这项研究为IAPP的结构与毒性之间的关系提供了新的思路,并指出了利用星形聚合物作为抗淀粉样蛋白生成的新型治疗剂的潜力。原子分子动力学模拟表明,PHEA臂可作为棒状支架用于IAPP结合,并随后通过增加局部肽浓度来加速IAPP聚集。发现星形纳米颗粒的三级结构对于驱动促进加速的IAPP聚集所需的特定相互作用至关重要。这项研究为IAPP的结构与毒性之间的关系提供了新的思路,并指出了利用星形聚合物作为抗淀粉样蛋白生成的新型治疗剂的潜力。原子分子动力学模拟显示,PHEA臂可作为棒状支架用于IAPP结合,并随后通过增加局部肽浓度来加速IAPP聚集。发现星形纳米颗粒的三级结构对于驱动促进加速的IAPP聚集所需的特定相互作用至关重要。这项研究为IAPP的结构与毒性之间的关系提供了新的思路,并指出了利用星形聚合物作为抗淀粉样蛋白生成的新型治疗剂的潜力。
更新日期:2017-10-31
中文翻译:

星状聚合物通过加速淀粉样蛋白聚集降低胰岛淀粉样蛋白多肽的毒性
在神经退行性疾病和2型糖尿病的整个谱中,蛋白质聚集到淀粉样蛋白原纤维中是普遍存在的现象。对抗淀粉样蛋白生成的常见策略是通过抑制小分子或纳米颗粒的蛋白质聚集来最大程度减少有毒寡聚物和原纤维的数量。然而,自然界中黑色素的合成是通过加速蛋白原纤化来规避有毒中间体的积累而实现的。因此,我们设计并证明了使用星形聚丙烯酸2-羟乙酯(PHEA)纳米结构促进聚集,同时改善人胰岛淀粉样多肽(IAPP)的毒性,参与血糖控制的肽和2型糖尿病的发病机理2糖尿病。PHEA的结合提高了IAPP聚集体中β-折叠的含量,同时呈现了源自聚合物的“固态”淀粉样蛋白的新形态。原子分子动力学模拟显示,PHEA臂可作为棒状支架用于IAPP结合,并随后通过增加局部肽浓度来加速IAPP聚集。发现星形纳米颗粒的三级结构对于驱动促进加速的IAPP聚集所需的特定相互作用至关重要。这项研究为IAPP的结构与毒性之间的关系提供了新的思路,并指出了利用星形聚合物作为抗淀粉样蛋白生成的新型治疗剂的潜力。原子分子动力学模拟表明,PHEA臂可作为棒状支架用于IAPP结合,并随后通过增加局部肽浓度来加速IAPP聚集。发现星形纳米颗粒的三级结构对于驱动促进加速的IAPP聚集所需的特定相互作用至关重要。这项研究为IAPP的结构与毒性之间的关系提供了新的思路,并指出了利用星形聚合物作为抗淀粉样蛋白生成的新型治疗剂的潜力。原子分子动力学模拟显示,PHEA臂可作为棒状支架用于IAPP结合,并随后通过增加局部肽浓度来加速IAPP聚集。发现星形纳米颗粒的三级结构对于驱动促进加速的IAPP聚集所需的特定相互作用至关重要。这项研究为IAPP的结构与毒性之间的关系提供了新的思路,并指出了利用星形聚合物作为抗淀粉样蛋白生成的新型治疗剂的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号