当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Metastable Form: A Route for the Generation of Hydrate and Anhydrous Forms of Ceritinib
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.cgd.7b01027 Ramanaiah Chennuru 1, 2 , Ravi Teja Koya 1 , Pavan Kommavarapu 1 , Saladi Venkata Narasayya 1 , Prakash Muthudoss 1 , Peddy Vishweshwar 1 , R. Ravi Chandra Babu 2 , Sudarshan Mahapatra 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.cgd.7b01027 Ramanaiah Chennuru 1, 2 , Ravi Teja Koya 1 , Pavan Kommavarapu 1 , Saladi Venkata Narasayya 1 , Prakash Muthudoss 1 , Peddy Vishweshwar 1 , R. Ravi Chandra Babu 2 , Sudarshan Mahapatra 1
Affiliation
|
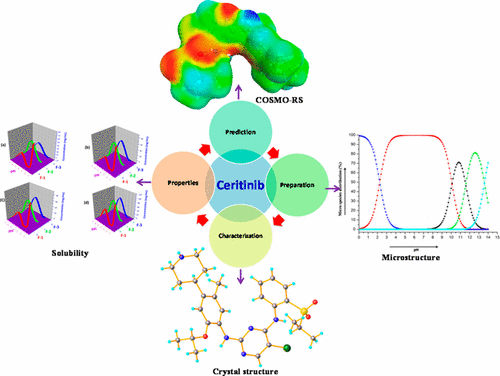
Ceritinib is an anaplastic lymphoma kinase (ALK) inhibitor used for the treatment of ALK-positive metastatic non-small cell lung cancer (NSCLC). This BCS class IV drug is developed by Novartis and traded under the name Zykadia. To date two forms [Form A (marketed form) and B] of ceritinib are disclosed in international patent application US 2013/0274279 A1. However, the crystal structure and insight into any solid form of this compound are not available in the literature. In order to achieve better physicochemical properties compared to known solid forms of this compound, novel polymorph identification is chosen as one of the challenging paths to address the issue. In our comprehensive polymorph screening, including in silico and experimental investigations, we discovered three novel solid forms of ceritinib. Out of these three solid forms, two are neat (Form 1 and Form 3) and the remaining one is a hydrate (Form 2). All synthesized forms are further characterized by powder X-ray diffraction, differential scanning calorimetry, and Fourier transform infrared spectroscopy. It is interesting to note that the discovery of this hydrate is in sync with the prediction done using COSMO-RS theory (COSMOthermX software). The current article includes the first single crystal structure of ceritinib Form 1. All forms (Form 1, 2, and 3) of ceritinib are subjected to physicochemical property evaluation like solubility in buffers with a pH range of 1–7, dissolution, and stability. In aqueous solutions and pH 4.5 (acetate buffer), the solubility of Form 2 and 3 is high compared to Form 1, whereas in 0.1 N HCl and 0.01 N HCl Form 1 has a higher solubility compared to Forms 2 and 3. A six-month stability study indicates that all forms (Forms 1, 2, and 3) are stable in ICH stability conditions like accelerated (40 °C ± 2 °C, 75% RH ± 5% RH), long-term (25 °C ± 2 °C, 60% RH ± 5% RH), and low temperature (2–8 °C) conditions. A thorough polymorph screening protocol, including in silico prediction, single crystal structure, and physicochemical properties of different forms and structure property correlations for ceritinib are enlightened in the current paper.
中文翻译:

原位亚稳形式:赛瑞替尼水合物和无水形式的生成途径
Ceritinib是一种间变性淋巴瘤激酶(ALK)抑制剂,用于治疗ALK阳性转移性非小细胞肺癌(NSCLC)。这种BCS IV类药物是由诺华公司开发,并以Zykadia的名称进行交易。迄今为止,在国际专利申请US 2013/0274279 A1中公开了ceritinib的两种形式[形式A(销售形式)和形式B]。但是,文献中没有该化合物的晶体结构和对任何固体形式的了解。为了与该化合物的已知固体形式相比获得更好的理化性质,选择新颖的多晶型物鉴定作为解决该问题的挑战性途径之一。在我们全面的多晶型物筛选中,包括计算机模拟和实验研究,我们发现了ceritinib的三种新型固体形式。在这三种固体形式中,两种是纯净的(形式1和形式3),其余的是水合物(形式2)。通过粉末X射线衍射,差示扫描量热法和傅里叶变换红外光谱进一步表征所有合成形式。有趣的是,该水合物的发现与使用COSMO-RS理论(COSMOthermX软件)所做的预测是同步的。当前文章包括ceritinib 1型的第一个单晶结构。对ceritinib的所有形式(1、2和3型)都进行了理化性质评估,例如在pH范围为1–7的缓冲液中的溶解度,溶解度和稳定性。在水溶液和pH 4.5(醋酸盐缓冲液)中,形式2和3的溶解度比形式1高,而在0.1 N HCl和0.01 N HCl中,形式1的溶解度比形式2和3高。六个月的稳定性研究表明,所有形式(表格1、2和3)在ICH稳定条件下均稳定,如加速(40°C±2°C,75%RH±5%RH),长期(25 °C±2°C,60%RH±5%RH)和低温(2–8°C)条件。本文对彻底的多晶型物筛选方案进行了启发,包括计算机模拟预测,单晶结构以及不同形式的理化性质以及塞立替尼的结构性质相关性。
更新日期:2017-10-31
中文翻译:

原位亚稳形式:赛瑞替尼水合物和无水形式的生成途径
Ceritinib是一种间变性淋巴瘤激酶(ALK)抑制剂,用于治疗ALK阳性转移性非小细胞肺癌(NSCLC)。这种BCS IV类药物是由诺华公司开发,并以Zykadia的名称进行交易。迄今为止,在国际专利申请US 2013/0274279 A1中公开了ceritinib的两种形式[形式A(销售形式)和形式B]。但是,文献中没有该化合物的晶体结构和对任何固体形式的了解。为了与该化合物的已知固体形式相比获得更好的理化性质,选择新颖的多晶型物鉴定作为解决该问题的挑战性途径之一。在我们全面的多晶型物筛选中,包括计算机模拟和实验研究,我们发现了ceritinib的三种新型固体形式。在这三种固体形式中,两种是纯净的(形式1和形式3),其余的是水合物(形式2)。通过粉末X射线衍射,差示扫描量热法和傅里叶变换红外光谱进一步表征所有合成形式。有趣的是,该水合物的发现与使用COSMO-RS理论(COSMOthermX软件)所做的预测是同步的。当前文章包括ceritinib 1型的第一个单晶结构。对ceritinib的所有形式(1、2和3型)都进行了理化性质评估,例如在pH范围为1–7的缓冲液中的溶解度,溶解度和稳定性。在水溶液和pH 4.5(醋酸盐缓冲液)中,形式2和3的溶解度比形式1高,而在0.1 N HCl和0.01 N HCl中,形式1的溶解度比形式2和3高。六个月的稳定性研究表明,所有形式(表格1、2和3)在ICH稳定条件下均稳定,如加速(40°C±2°C,75%RH±5%RH),长期(25 °C±2°C,60%RH±5%RH)和低温(2–8°C)条件。本文对彻底的多晶型物筛选方案进行了启发,包括计算机模拟预测,单晶结构以及不同形式的理化性质以及塞立替尼的结构性质相关性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号