当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibacterial 3,6-Disubstituted 4-Hydroxy-5,6-dihydro-2H-pyran-2-ones from Serratia plymuthica MF371-2
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00565 Joakim Bjerketorp 1 , Jolanta J. Levenfors 1 , Christer Sahlberg 2 , Christina L. Nord 1 , Pierre F. Andersson 1 , Bengt Guss 3 , Bo Öberg 4, 5 , Anders Broberg 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00565 Joakim Bjerketorp 1 , Jolanta J. Levenfors 1 , Christer Sahlberg 2 , Christina L. Nord 1 , Pierre F. Andersson 1 , Bengt Guss 3 , Bo Öberg 4, 5 , Anders Broberg 1
Affiliation
|
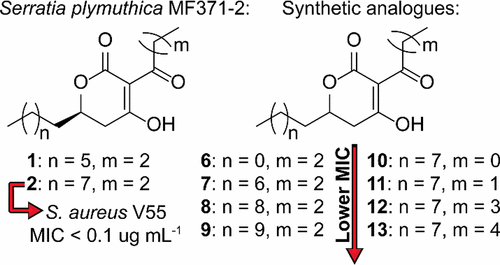
Bioassay-guided fractionation of culture extracts of Serratia plymuthica strain MF371-2 resulted in the isolation of two new antibacterial compounds with potent activity against Gram-positive bacteria, including Staphylococcus aureus LMG 15975 (MRSA). A spectroscopic investigation, in combination with synthesis, enabled the characterization of the compounds as 3-butyryl-4-hydroxy-6-heptyl-5,6-dihydro-2H-pyran-2-one (plymuthipyranone A, 1) and 3-butyryl-4-hydroxy-6-nonyl-5,6-dihydro-2H-pyran-2-one (plymuthipyranone B, 2). The MIC values for 1 and 2 against S. aureus LMG 15975 were determined to be 1–2 μg mL–1 and 0.8 μg mL–1, respectively. Compound 2 was found to have potent activity against many strains of S. aureus, including several mupirocin-resistant strains, other species of Staphylococcus, and vancomycin-resistant enterococci. Compound 2 was slightly cytotoxic for human cells, with CC50 values between 4.7 and 40 μg mL–1, but the CC50/MIC ratio was ≥10 for many tested combinations of human cells and bacteria, suggesting its possible use as an antibacterial agent. Several analogues were synthesized with different alkyl groups in the 3- and 6-positions (6–13), and their biological properties were evaluated. It was concluded that the activity of the compounds increased with the lengths of the alkyl and acyl substituents.
中文翻译:

沙雷氏菌MF371-2的抗菌3,6-二取代的4-羟基-5,6-二氢-2 H-吡喃-2-酮
生物测定指导的粘膜沙雷氏菌MF371-2菌株培养物提取物的分离导致分离出两种对革兰氏阳性细菌具有有效活性的新型抗菌化合物,包括金黄色葡萄球菌LMG 15975(MRSA)。光谱研究与合成相结合,能够将化合物表征为3-butyryl-4-hydroxy-6- heptyl -5,6-dihydro-2 H -pyran-2-one(plymuthipyranone A,1)和3 -丁酰基-4-羟基-6-壬基-5,6-二氢-2 H-吡喃-2-酮(plymuthipyranone B,2)。1和2对金黄色葡萄球菌LMG 15975的MIC值确定为1-2μgmL分别为–1和0.8μgmL –1。发现化合物2对许多金黄色葡萄球菌菌株具有有效的活性,包括几个对莫匹罗星耐药的菌株,其他葡萄球菌和对万古霉素耐药的肠球菌。化合物2对人体细胞具有轻微的细胞毒性,CC 50值在4.7至40μgmL –1之间,但对于许多经过测试的人体细胞和细菌组合,CC 50 / MIC比率均≥10,表明它可能用作抗菌剂。合成了一些类似的类似物,这些类似物在3和6位上具有不同的烷基(6 – 13),并对其生物学特性进行了评估。结论是,化合物的活性随烷基和酰基取代基的长度增加而增加。
更新日期:2017-10-30
中文翻译:

沙雷氏菌MF371-2的抗菌3,6-二取代的4-羟基-5,6-二氢-2 H-吡喃-2-酮
生物测定指导的粘膜沙雷氏菌MF371-2菌株培养物提取物的分离导致分离出两种对革兰氏阳性细菌具有有效活性的新型抗菌化合物,包括金黄色葡萄球菌LMG 15975(MRSA)。光谱研究与合成相结合,能够将化合物表征为3-butyryl-4-hydroxy-6- heptyl -5,6-dihydro-2 H -pyran-2-one(plymuthipyranone A,1)和3 -丁酰基-4-羟基-6-壬基-5,6-二氢-2 H-吡喃-2-酮(plymuthipyranone B,2)。1和2对金黄色葡萄球菌LMG 15975的MIC值确定为1-2μgmL分别为–1和0.8μgmL –1。发现化合物2对许多金黄色葡萄球菌菌株具有有效的活性,包括几个对莫匹罗星耐药的菌株,其他葡萄球菌和对万古霉素耐药的肠球菌。化合物2对人体细胞具有轻微的细胞毒性,CC 50值在4.7至40μgmL –1之间,但对于许多经过测试的人体细胞和细菌组合,CC 50 / MIC比率均≥10,表明它可能用作抗菌剂。合成了一些类似的类似物,这些类似物在3和6位上具有不同的烷基(6 – 13),并对其生物学特性进行了评估。结论是,化合物的活性随烷基和酰基取代基的长度增加而增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号