Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-10-27 , DOI: 10.1016/j.tetlet.2017.10.072 Shuji Kyokane , Yusuke Tanaka , Yoshihisa Sei , Masashi Shiotsuki
|
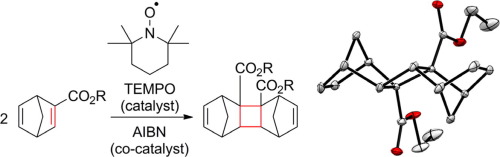
An organocatalytic [2+2] cycloaddition reaction of norbornadienes (NBDs) using catalytic amount of TEMPO was reported. Single crystal X-ray diffraction of the product revealed its detailed multicyclic structure containing a 4-membered ring, formed in intermolecular reaction. Addition of AIBN to the current catalytic system improved the product yield. Quantitative reaction of the NBD and TEMPO gave a 2:2 adduct of NBD and TEMPO, which was confirmed by HR-MS. This catalytic [2+2] addition of NBDs has great advantage in selective intermolecular coupling in comparison with [2+2] photocycloaddition.
中文翻译:

稳定的有机自由基化合物对降冰片二烯的有机催化分子间[2 + 2]环加成反应
报道了使用催化量的TEMPO进行降冰片二烯(NBD)的有机催化[2 + 2]环加成反应。该产物的单晶X射线衍射揭示了其详细的多环结构,该多环结构包含在分子间反应中形成的4元环。在当前的催化体系中添加AIBN可以提高产品收率。NBD和TEMPO的定量反应产生了NBD和TEMPO的2:2加合物,这已通过HR-MS证实。与[2 + 2]光环加成相比,这种NBD的催化[2 + 2]加成在选择性分子间偶联方面具有很大优势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号