Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-10-27 , DOI: 10.1016/j.apcatb.2017.10.061 Anna Krukowska , Michal Jerzy Winiarski , Judyta Strychalska-Nowak , Tomasz Klimczuk , Wojciech Lisowski , Alicja Mikolajczyk , Henry P. Pinto , Tomasz Puzyn , Tomasz Grzyb , Adriana Zaleska-Medynska
|
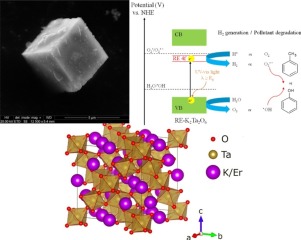
Novel rare earth-doped K2Ta2O6 (RE-K2Ta2O6) photocatalysts were successfully synthesized by one-step hydrothermal method. The effect of dopant type (RE = Y, Yb, Ho, Pr, Er) and amount of rare earth precursor (2, 4, 8 and 10 mol%) on the physicochemical and photocatalytic properties of RE-K2Ta2O6 have been investigated. All as-prepared materials were subsequently characterized by UV–vis diffuse reflectance spectroscopy (DRS), Brunauer-Emmett-Teller (BET) specific surface area measurement, scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDS), powder X-ray diffraction (PXRD), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, mass magnetic susceptometry and photoluminescence (PL) emission spectroscopy. The photocatalytic activity under UV–vis light irradiation was estimated in phenol degradation in aqueous phase, toluene removal in gas phase and H2 generation from formic acid solution. The experimental results show that, novel RE-K2Ta2O6 exhibits greatly improved degradation efficiency under UV–vis light irradiation compared with pristine K2Ta2O6. The Er-K2Ta2O6 and Pr-K2Ta2O6, obtained by introducing of 2 mol% of RE ions during synthesis, reveal the highest photocatalytic activity among prepared samples in aqueous phase (33% of phenol decomposition after 90 min of irradiation) and gas phase (45% of toluene removal after 60 min of irradiation), respectively. Moreover, both photocatalysts present good stability after subsequent three cycles. The active species trapping test shows that OH and O2
− radicals are significantly involved in phenol oxidation under UV–vis light irradiation. The amount of H2 evolution increases with increasing addition of Er dopant into K2Ta2O6 lattice. The highest H2 production is obtained for 10 mol% Er-K2Ta2O6 after 240 min of UV–vis light irradiation (15.40 μmol/min). Enhanced photoactivity performance can be attributed to incorporation of RE ions at K+ lattice site in RE-K2Ta2O6, probably leading to formation of new RE 4f states below the conduction band of K2Ta2O6 structure. To investigate the localization of RE ions in K2Ta2O6 structure, the band structure and partial density of the states (PDOS) have been investigated. Computer simulations were performed using plane-wave based Vienna ab-initio simulation package (VASP) with the generalized gradient approximation (GGA) by Perdew-Burke-Ernzerhof (PBE). Moreover, inclusion of RE ions in K2Ta2O6 causes predominance pyrochlore phase formation over perovskite in regular cubic structure. Summarized, RE-doped K2Ta2O6 is promising material in photocatalytic degradation of organic pollutants and H2 generation processes. Our work may provide valuable information for rare earth doping semiconductor with improved photocatalytic performance.
中文翻译:

稀土离子掺杂的K 2 Ta 2 O 6光催化剂具有增强的紫外可见光活性
通过一步水热法成功合成了新型稀土掺杂的K 2 Ta 2 O 6(RE-K 2 Ta 2 O 6)光催化剂。掺杂剂类型(RE = Y,Yb,Ho,Pr,Er)和稀土前体的量(2、4、8和10 mol%)对RE-K 2 Ta 2 O 6的物理化学和光催化性能的影响已被调查。随后通过紫外可见漫反射光谱法(DRS),布鲁瑙尔-埃米特-泰勒(BET)比表面积测量,扫描电子显微镜(SEM)和能量色散X射线光谱(EDS),粉末对所有制备的材料进行表征X射线衍射(PXRD),X射线光电子能谱(XPS),拉曼光谱,质量磁化法和光致发光(PL)发射光谱。在紫外可见光照射下的光催化活性估计为水相中的苯酚降解,气相中的甲苯去除和甲酸溶液中的H 2生成。实验结果表明,新型RE-K 2 Ta 2 O 6与原始的K 2 Ta 2 O 6相比,在紫外可见光照射下具有显着改善的降解效率。通过在合成过程中引入2 mol%的RE离子获得的Er-K 2 Ta 2 O 6和Pr-K 2 Ta 2 O 6,在水相中制备的样品中显示出最高的光催化活性(33%的苯酚分解后,照射90分钟)和气相(照射60分钟后除去45%的甲苯)。此外,两种光催化剂在随后的三个循环后均表现出良好的稳定性。活性种捕获测试表明,OH和O 2
-自由基在紫外可见光照射下显着参与苯酚氧化。随着向K 2 Ta 2 O 6晶格中添加Er掺杂剂的增加,H 2析出的量增加。紫外可见光照射240分钟(15.40μmol/ min)后,对于10 mol%的Er-K 2 Ta 2 O 6可获得最高的H 2生成量。增强的光敏性能可归因于RE离子在RE-K 2 Ta 2 O 6中的K +晶格位处掺入,可能导致在K 2 Ta导带以下形成新的RE 4f态。2 O 6结构。为了研究RE离子在K 2 Ta 2 O 6结构中的定位,研究了能带结构和部分态密度(PDOS)。使用基于平面波的维也纳ab-initio模拟软件包(VASP)和Perdew-Burke-Ernzerhof(PBE)的广义梯度近似(GGA)进行计算机模拟。此外,RE离子在K 2 Ta 2 O 6中的引入导致规则晶格结构中钙钛矿上主要的烧绿石相形成。总结,稀土掺杂的K 2 Ta 2 O 6在有机污染物的光催化降解和H 2生成过程中是很有前途的材料。我们的工作可能会为具有改善的光催化性能的稀土掺杂半导体提供有价值的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号