当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrogenerated Cationic Reactive Intermediates: The Pool Method and Further Advances
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.chemrev.7b00475 Jun-ichi Yoshida 1 , Akihiro Shimizu 1 , Ryutaro Hayashi 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.chemrev.7b00475 Jun-ichi Yoshida 1 , Akihiro Shimizu 1 , Ryutaro Hayashi 1
Affiliation
|
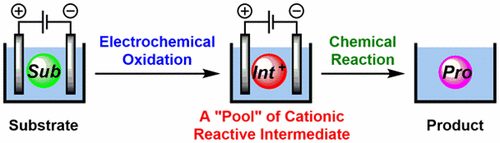
Electrochemistry serves as a powerful method for generating reactive intermediates, such as organic cations. In general, there are two ways to use reactive intermediates for chemical reactions: (1) generation in the presence of a reaction partner and (2) generation in the absence of a reaction partner with accumulation in solution as a “pool” followed by reaction with a subsequently added reaction partner. The former approach is more popular because reactive intermediates are usually short-lived transient species, but the latter method is more flexible and versatile. This review focuses on the latter approach and provides a concise overview of the current methods for the generation and accumulation of cationic reactive intermediates as a pool using modern techniques of electrochemistry and their reactions with subsequently added nucleophilic reaction partners.
中文翻译:

电生成的阳离子反应中间体:池法和进一步的进展
电化学是产生反应性中间体(例如有机阳离子)的有力方法。通常,有两种方法可以使用反应性中间体进行化学反应:(1)在存在反应伙伴的情况下生成,以及(2)在不存在反应伙伴的情况下生成,并在溶液中积累为“池”,然后进行反应与随后添加的反应伙伴。前一种方法更受欢迎,因为反应性中间体通常是短寿命的瞬态物种,而后一种方法则更加灵活和通用。
更新日期:2017-10-27
中文翻译:

电生成的阳离子反应中间体:池法和进一步的进展
电化学是产生反应性中间体(例如有机阳离子)的有力方法。通常,有两种方法可以使用反应性中间体进行化学反应:(1)在存在反应伙伴的情况下生成,以及(2)在不存在反应伙伴的情况下生成,并在溶液中积累为“池”,然后进行反应与随后添加的反应伙伴。前一种方法更受欢迎,因为反应性中间体通常是短寿命的瞬态物种,而后一种方法则更加灵活和通用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号