当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Short-Lived Ikaite Affects Calcite Crystallization
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acs.cgd.7b00743 R. Besselink 1 , J. D. Rodriguez-Blanco 2, 3 , T. M. Stawski 1 , L. G. Benning 1, 4, 5 , D. J. Tobler 2
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acs.cgd.7b00743 R. Besselink 1 , J. D. Rodriguez-Blanco 2, 3 , T. M. Stawski 1 , L. G. Benning 1, 4, 5 , D. J. Tobler 2
Affiliation
|
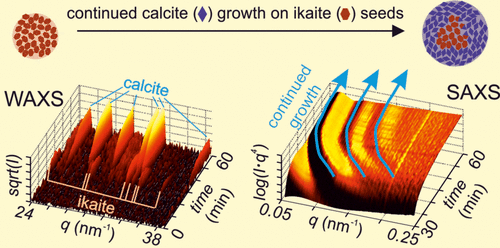
The pathways of CaCO3 crystallization are manifold, often involving one or several metastable amorphous or nanocrystalline intermediate phases. The presence of such intermediates is often overlooked, because they are short-lived and/or occur at small molar fractions. However, their occurrence does not just impact the mechanisms and pathways of formation of the final stable CaCO3 phase, but also affects their crystal size, shape, and structure. Here we document the presence of a short-lived intermediate through in situ and time-resolved small and wide-angle X-ray scattering combined with high resolution electron microscope observations. When ikaite forms concomitant with the dissolution of amorphous calcium carbonate (ACC) but prior to calcite formation, fairly large glendonite-type calcite crystals grow despite the presence of citrate ligands that usually reduce crystal size. These were ideal seeding crystals for further crystallization from supersaturated ions in solution. In contrast, in the absence of ikaite the crystallization of calcite proceeds through transformation from ACC, resulting in fine-grained spherulitic calcite with sizes ∼8 times smaller than when ikaite was present. Noteworthy is that the formation of the intermediate ikaite, although it consumes less than 3 mol % of the total precipitated CaCO3, still clearly affected the calcite formation mechanism.
中文翻译:

短寿命的岩浆石如何影响方解石结晶
CaCO 3结晶的途径是多方面的,通常涉及一个或几个亚稳态的无定形或纳米晶体中间相。这种中间体的存在常常被忽略,因为它们是短寿命的和/或以小摩尔分数存在。然而,它们的出现不仅影响最终稳定的CaCO 3相形成的机理和途径,而且还影响其晶体尺寸,形状和结构。在这里,我们通过原位记录了短寿命中间体的存在以及时间分辨的小和广角X射线散射与高分辨率电子显微镜观察相结合。当方解石形成伴随无定形碳酸钙(ACC)溶解但在方解石形成之前,尽管存在通常会减小晶体尺寸的柠檬酸盐配体,但仍会生成相当大的方钠石型方解石晶体。这些是理想的晶种,可用于从溶液中的过饱和离子进一步结晶。相比之下,在没有钾长石的情况下,方解石的结晶是通过ACC的转变而进行的,从而导致了细粒球形方解石的形成,其粒径是存在钾长石时的约8倍。值得注意的是,尽管形成的钾长石的消耗量少于总沉淀的CaCO 3的3 mol%,但形成了中间的钾长石,仍然清楚地影响了方解石的形成机理。
更新日期:2017-10-26
中文翻译:

短寿命的岩浆石如何影响方解石结晶
CaCO 3结晶的途径是多方面的,通常涉及一个或几个亚稳态的无定形或纳米晶体中间相。这种中间体的存在常常被忽略,因为它们是短寿命的和/或以小摩尔分数存在。然而,它们的出现不仅影响最终稳定的CaCO 3相形成的机理和途径,而且还影响其晶体尺寸,形状和结构。在这里,我们通过原位记录了短寿命中间体的存在以及时间分辨的小和广角X射线散射与高分辨率电子显微镜观察相结合。当方解石形成伴随无定形碳酸钙(ACC)溶解但在方解石形成之前,尽管存在通常会减小晶体尺寸的柠檬酸盐配体,但仍会生成相当大的方钠石型方解石晶体。这些是理想的晶种,可用于从溶液中的过饱和离子进一步结晶。相比之下,在没有钾长石的情况下,方解石的结晶是通过ACC的转变而进行的,从而导致了细粒球形方解石的形成,其粒径是存在钾长石时的约8倍。值得注意的是,尽管形成的钾长石的消耗量少于总沉淀的CaCO 3的3 mol%,但形成了中间的钾长石,仍然清楚地影响了方解石的形成机理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号