当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd‐Catalyzed versus Uncatalyzed, PhI(OAc)2‐Mediated Cyclization Reactions of N6‐([1,1′‐Biaryl]‐2‐yl)Adenine Nucleosides
ChemCatChem ( IF 3.8 ) Pub Date : 2017-10-24 , DOI: 10.1002/cctc.201700918 Sakilam Satishkumar 1 , Suresh Poudapally 2 , Prasanna K. Vuram 1 , Venkateshwarlu Gurram 2 , Narender Pottabathini 2 , Dellamol Sebastian 1, 3 , Lijia Yang 1 , Padmanava Pradhan 1 , Mahesh K. Lakshman 1, 3
ChemCatChem ( IF 3.8 ) Pub Date : 2017-10-24 , DOI: 10.1002/cctc.201700918 Sakilam Satishkumar 1 , Suresh Poudapally 2 , Prasanna K. Vuram 1 , Venkateshwarlu Gurram 2 , Narender Pottabathini 2 , Dellamol Sebastian 1, 3 , Lijia Yang 1 , Padmanava Pradhan 1 , Mahesh K. Lakshman 1, 3
Affiliation
|
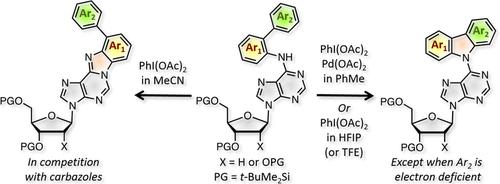
In this work we have assessed reactions of N6‐([1,1′‐biaryl]‐2‐yl)adenine nucleosides with Pd(OAc)2 and PhI(OAc)2, via a PdII/PdIV redox cycle. The substrates are readily obtained by Pd/Xantphos‐catalyzed reaction of adenine nucleosides with 2‐bromo‐1,1′‐biaryls. In PhMe, the N6‐biarylyl nucleosides gave C6‐carbazolyl nucleoside analogues by C−N bond formation with the exocyclic N6 nitrogen atom. In the solvent screening for the Pd‐catalyzed reactions, an uncatalyzed process was found to be operational. It was observed that the carbazolyl products could also be obtained in the absence of a metal catalyst by reaction with PhI(OAc)2 in 1,1,1,3,3,3‐hexafluoroisopropanol (HFIP). Thus, under Pd catalysis and in HFIP, reactions proceed to provide carbazolyl nucleoside analogues, with some differences. If reactions of N6‐biarylyl nucleoside substrates were conducted in MeCN, formation of aryl benzimidazopurinyl nucleoside derivatives was observed in many cases by C−N bond formation with the N1 ring nitrogen atom of the purine (carbazole and benzimidazole isomers are readily separated by chromatography). Whereas PdII/PdIV redox is responsible for carbazole formation under the metal‐catalyzed conditions, in HFIP and MeCN radical cations and/or nitrenium ions can be intermediates. An extensive set of radical inhibition experiments was conducted and the data are presented.
中文翻译:

Pd催化与未催化的PhI(OAc)2介导的N6-([1,1'-Biaryl] -2-yl)腺嘌呤核苷环化反应
在这项工作中,我们通过Pd II / Pd IV氧化还原循环评估了N 6 -([1,1'-联芳基] -2-基)腺嘌呤核苷与Pd(OAc)2和PhI(OAc)2的反应。底物很容易通过Pd / Xantphos催化的腺嘌呤核苷与2-bromo-1,1'-biaryls的反应而获得。在PhMe中,N 6-联芳基核苷通过与环外N 6的C-N键形成而得到C6-咔唑基核苷类似物氮原子。在Pd催化反应的溶剂筛选中,发现未催化的过程是可行的。据观察,咔唑基产物也可以在不存在金属催化剂的情况下,通过与PhI(OAc)2在1,1,1,3,3,3-六氟异丙醇(HFIP)中反应而获得。因此,在Pd催化下和在HFIP中,反应进行提供了咔唑基核苷类似物,但有一些区别。如果在MeCN中进行N 6-联芳基核苷底物的反应,则在许多情况下通过与嘌呤的N 1环氮原子形成CN键可观察到芳基苯并咪唑嘌呤基核苷衍生物的形成(咔唑和苯并咪唑异构体很容易通过色谱)。而Pd II/ Pd IV氧化还原负责金属催化条件下咔唑的形成,在HFIP和MeCN自由基阳离子和/或or离子可以是中间体。进行了大量的自由基抑制实验,并给出了数据。
更新日期:2017-10-24
中文翻译:

Pd催化与未催化的PhI(OAc)2介导的N6-([1,1'-Biaryl] -2-yl)腺嘌呤核苷环化反应
在这项工作中,我们通过Pd II / Pd IV氧化还原循环评估了N 6 -([1,1'-联芳基] -2-基)腺嘌呤核苷与Pd(OAc)2和PhI(OAc)2的反应。底物很容易通过Pd / Xantphos催化的腺嘌呤核苷与2-bromo-1,1'-biaryls的反应而获得。在PhMe中,N 6-联芳基核苷通过与环外N 6的C-N键形成而得到C6-咔唑基核苷类似物氮原子。在Pd催化反应的溶剂筛选中,发现未催化的过程是可行的。据观察,咔唑基产物也可以在不存在金属催化剂的情况下,通过与PhI(OAc)2在1,1,1,3,3,3-六氟异丙醇(HFIP)中反应而获得。因此,在Pd催化下和在HFIP中,反应进行提供了咔唑基核苷类似物,但有一些区别。如果在MeCN中进行N 6-联芳基核苷底物的反应,则在许多情况下通过与嘌呤的N 1环氮原子形成CN键可观察到芳基苯并咪唑嘌呤基核苷衍生物的形成(咔唑和苯并咪唑异构体很容易通过色谱)。而Pd II/ Pd IV氧化还原负责金属催化条件下咔唑的形成,在HFIP和MeCN自由基阳离子和/或or离子可以是中间体。进行了大量的自由基抑制实验,并给出了数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号