当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of an Electrochemical Method to Evaluation of Amyloid-β Aggregation Inhibitors: Testing the RGKLVFFGR-NH2 Peptide Antiaggregant
Electroanalysis ( IF 2.7 ) Pub Date : 2017-10-25 , DOI: 10.1002/elan.201700499 Elena V. Suprun 1 , Sergey P. Radko 1 , Tatiana E. Farafonova 1 , Vladimir A. Mitkevich 2 , Alexander A. Makarov 2 , Alexander I. Archakov 1 , Victoria V. Shumyantseva 1
Electroanalysis ( IF 2.7 ) Pub Date : 2017-10-25 , DOI: 10.1002/elan.201700499 Elena V. Suprun 1 , Sergey P. Radko 1 , Tatiana E. Farafonova 1 , Vladimir A. Mitkevich 2 , Alexander A. Makarov 2 , Alexander I. Archakov 1 , Victoria V. Shumyantseva 1
Affiliation
|
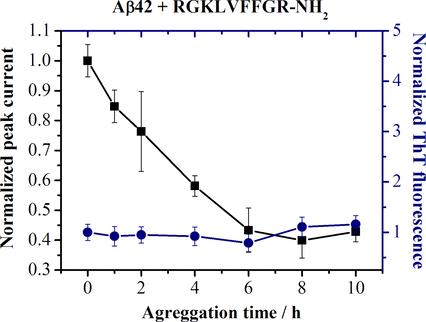
The aggregation of amyloid-β peptide (Aβ) is believed to play a crucial role in the Alzheimer's disease (AD) pathogenesis and is considered as a therapeutic target for treating AD. The Aβ electrooxidation via a Tyr-10 residue, sensitive to a depletion of a pool of Aβ monomers and oligomers in the course of Aβ aggregation, may be employed for testing natural and synthetic organic compounds (including short peptides) potentially able to inhibit the pathological Aβ aggregation (antiaggregants). In the present work, using the known peptide antiaggregant RGKLVFFGR-NH2 (OR2) and its scrambled variant KGLRVGFRF-NH2 as a control, we demonstrate that the electrochemical method based on electrooxidation of an Aβ42 Tyr-10 residue, when combined with methods allowing for the evaluation of the Aβ42 aggregate structure and size, can provide essential information regarding the antiaggregant impact on Aβ42 aggregation. Electrochemical measurements were performed using square wave voltammetry on carbon screen printed electrodes whereas the Aβ42 aggregate structure and size were analyzed by means of the conventional thioflavin T (ThT) based fluorescence assay and dynamic light scattering. While inhibiting Aβ42 fibrillation as manifested by the unchanged level of ThT fluorescence, the OR2 peptide antiaggregant had no effect on the decrease of Aβ42 electrooxidation current in the course of Aβ42 aggregation. These observations suggest that OR2 does not stop the aggregation but redirects it into a pathway where amorphous rather than fibrillar aggregates are formed. Hence, the direct electrochemistry appears to offer a simple and cost-effective approach for probing potential peptide antiaggregants, which is complementary to methods based on detecting Aβ aggregates.
中文翻译:

应用电化学方法评估淀粉样蛋白-β 聚集抑制剂:测试 RGKLVFFGR-NH2 肽抗聚集剂
β 淀粉样肽 (Aβ) 的聚集被认为在阿尔茨海默病 (AD) 的发病机制中起关键作用,被认为是治疗 AD 的治疗靶点。通过 Tyr-10 残基进行的 Aβ 电氧化,对 Aβ 聚集过程中 Aβ 单体和寡聚体库的消耗很敏感,可用于测试天然和合成有机化合物(包括短肽),可能能够抑制病理学Aβ 聚集(抗聚集剂)。在目前的工作中,使用已知的肽抗聚集剂 RGKLVFFGR-NH2 (OR2) 及其混杂变体 KGLRVGFRF-NH2 作为对照,我们证明了基于 Aβ42 Tyr-10 残基电氧化的电化学方法,当与允许Aβ42 聚集体结构和大小的评估,可以提供有关抗聚集剂对 Aβ42 聚集影响的重要信息。电化学测量是在碳丝网印刷电极上使用方波伏安法进行的,而 Aβ42 聚集体的结构和尺寸则通过基于传统硫代黄素 T (ThT) 的荧光测定和动态光散射进行分析。在抑制 Aβ42 纤颤的同时,如 ThT 荧光水平不变,OR2 肽抗聚集剂对 Aβ42 聚集过程中 Aβ42 电氧化电流的降低没有影响。这些观察结果表明 OR2 不会阻止聚集,而是将其重定向到形成无定形而不是纤维状聚集体的途径。因此,
更新日期:2017-10-25
中文翻译:

应用电化学方法评估淀粉样蛋白-β 聚集抑制剂:测试 RGKLVFFGR-NH2 肽抗聚集剂
β 淀粉样肽 (Aβ) 的聚集被认为在阿尔茨海默病 (AD) 的发病机制中起关键作用,被认为是治疗 AD 的治疗靶点。通过 Tyr-10 残基进行的 Aβ 电氧化,对 Aβ 聚集过程中 Aβ 单体和寡聚体库的消耗很敏感,可用于测试天然和合成有机化合物(包括短肽),可能能够抑制病理学Aβ 聚集(抗聚集剂)。在目前的工作中,使用已知的肽抗聚集剂 RGKLVFFGR-NH2 (OR2) 及其混杂变体 KGLRVGFRF-NH2 作为对照,我们证明了基于 Aβ42 Tyr-10 残基电氧化的电化学方法,当与允许Aβ42 聚集体结构和大小的评估,可以提供有关抗聚集剂对 Aβ42 聚集影响的重要信息。电化学测量是在碳丝网印刷电极上使用方波伏安法进行的,而 Aβ42 聚集体的结构和尺寸则通过基于传统硫代黄素 T (ThT) 的荧光测定和动态光散射进行分析。在抑制 Aβ42 纤颤的同时,如 ThT 荧光水平不变,OR2 肽抗聚集剂对 Aβ42 聚集过程中 Aβ42 电氧化电流的降低没有影响。这些观察结果表明 OR2 不会阻止聚集,而是将其重定向到形成无定形而不是纤维状聚集体的途径。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号