当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthesis of (−)-5-Hydroxy-equol and 5-Hydroxy-dehydroequol from Soy Isoflavone, Genistein Using Microbial Whole Cell Bioconversion
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/acschembio.7b00624 Pyung-Gang Lee 1, 2 , Joonwon Kim 1, 2 , Eun-Jung Kim 2 , Sang-Hyuk Lee 1, 2 , Kwon-Young Choi 3 , Romas J. Kazlauskas 4 , Byung-Gee Kim 1, 2, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/acschembio.7b00624 Pyung-Gang Lee 1, 2 , Joonwon Kim 1, 2 , Eun-Jung Kim 2 , Sang-Hyuk Lee 1, 2 , Kwon-Young Choi 3 , Romas J. Kazlauskas 4 , Byung-Gee Kim 1, 2, 5
Affiliation
|
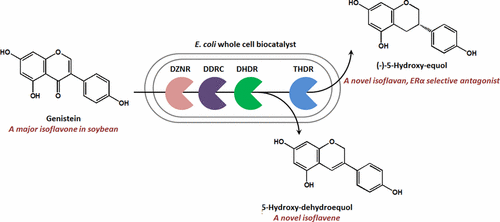
Equols are isoflavandiols formed by reduction of soy isoflavones such as daidzein and genistein by gut microorganisms. These phytoestrogens are of interest for their various biological effects. We report biosynthesis from genistein to (−)-5-hydroxy-equol in recombinant E. coli expressing three reductases (daidzein reductase DZNR, dihidrodaidzein reductase DHDR, tetrahydrodaidzein reductase THDR) and a racemase (dihydrodaidzein racemase, DDRC) originating from the gut bacterium, Slackia isoflavoniconvertens. The biosynthesized 5-hydroxy-equol proved as an optically negative enantiomer, nonetheless it displayed an inverse circular dichroism spectrum to (S)-equol. Compartmentalized expression of DZNR and DDRC in one E. coli strain and DHDR and THDR in another increased the yield to 230 mg/L and the productivity to 38 mg/L/h. If the last reductase was missing, the intermediate spontaneously dehydrated to 5-hydroxy-dehydroequol in up to 99 mg/L yield. This novel isoflavene, previously not known to be synthesized in nature, was also detected in this biotransformation system. Although (S)-equol favors binding to human estrogen receptor (hER) β over hERα, (−)-5-hydroxy-equol showed the opposite preference. This study provides elucidation of the biosynthetic route of (−)-5-hydroxy-equol and measurement of its potent antagonistic character as a phytoestrogen for the first time.
中文翻译:

微生物全细胞生物转化法从大豆异黄酮中合成(-)-5-羟基-雌马酚和5-羟基-脱氢雌马酚
雌二醇是通过肠道微生物还原大豆异黄酮(如大豆苷元和染料木黄酮)而形成的异黄烷二醇。这些植物雌激素因其多种生物学作用而受到关注。我们报告了从染料木黄酮到重组大肠杆菌的(-)-5-羟基-雌马酚的生物合成,表达三种还原酶(黄豆苷元还原酶DZNR,双氢黄豆苷元还原酶DHDR,四氢黄豆苷元还原酶THDR)和消旋酶(二氢黄豆苷元消旋酶,DDRC)。 ,Slackia isoflavoniconvertens。经生物合成的5-羟基-雌马酚被证明是光学负性对映体,尽管如此,它仍显示出与(S)-雌马酚相反的圆二色性光谱。一株大肠杆菌中DZNR和DDRC的区室表达菌株和DHDR和THDR在另一个条件下将产量提高到230 mg / L,将生产率提高到38 mg / L / h。如果缺少最后一个还原酶,则中间体会自发脱水成5-羟基-脱氢雌马酚,产率高达99 mg / L。在这种生物转化系统中还检测到了这种新型的异黄酮,以前未知的是天然合成的。尽管(S)-雌马酚比hERα更倾向于与人雌激素受体(hER)β结合,但(-)-5-羟基-雌马酚显示出相反的偏好。这项研究首次阐明了(-)-5-羟基雌马酚的生物合成途径,并首次测量了其作为植物雌激素的强拮抗特性。
更新日期:2017-10-23
中文翻译:

微生物全细胞生物转化法从大豆异黄酮中合成(-)-5-羟基-雌马酚和5-羟基-脱氢雌马酚
雌二醇是通过肠道微生物还原大豆异黄酮(如大豆苷元和染料木黄酮)而形成的异黄烷二醇。这些植物雌激素因其多种生物学作用而受到关注。我们报告了从染料木黄酮到重组大肠杆菌的(-)-5-羟基-雌马酚的生物合成,表达三种还原酶(黄豆苷元还原酶DZNR,双氢黄豆苷元还原酶DHDR,四氢黄豆苷元还原酶THDR)和消旋酶(二氢黄豆苷元消旋酶,DDRC)。 ,Slackia isoflavoniconvertens。经生物合成的5-羟基-雌马酚被证明是光学负性对映体,尽管如此,它仍显示出与(S)-雌马酚相反的圆二色性光谱。一株大肠杆菌中DZNR和DDRC的区室表达菌株和DHDR和THDR在另一个条件下将产量提高到230 mg / L,将生产率提高到38 mg / L / h。如果缺少最后一个还原酶,则中间体会自发脱水成5-羟基-脱氢雌马酚,产率高达99 mg / L。在这种生物转化系统中还检测到了这种新型的异黄酮,以前未知的是天然合成的。尽管(S)-雌马酚比hERα更倾向于与人雌激素受体(hER)β结合,但(-)-5-羟基-雌马酚显示出相反的偏好。这项研究首次阐明了(-)-5-羟基雌马酚的生物合成途径,并首次测量了其作为植物雌激素的强拮抗特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号