Dyes and Pigments ( IF 4.1 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.dyepig.2017.10.006 Xin-Long Sha , Jin-Wei Xiao , De-Hang Yin , Ru Sun , Yu-Jie Xu , Jian-Feng Ge
|
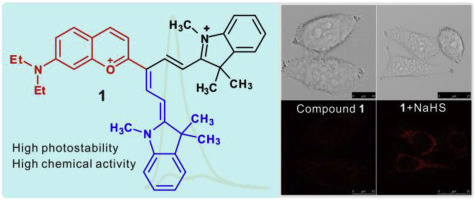
The cyanine dye (1) with three arms of one chromenyl group and two indol groups was synthesized in this paper; and the proposed synthetic mechanism was also mentioned. It has excellent photostability compared to three typical cyanine dyes. Dye 1 gives high chemical activity and nearly turn-on fluorescent response towards hydrosulfide at 600–800 nm, it also has good selectivity towards hydrosulfide over other species; the detection reaction mechanism was proved by 1H NMR analysis, and the emission responses was elucidated by time dependent density functional theory (TDDFT) calculation. The in cellular fluorescent imaging application of the dye 1 was successfully performed for the hydrosulfide imaging of HeLa cells.
中文翻译:

一种光稳定的Tribrachia花青染料的制备及其对氢硫化物的高化学活性
合成了具有一个色烯基和两个吲哚基的三臂的花青染料(1);还提到了拟议的综合机制。与三种典型的花青染料相比,它具有出色的光稳定性。染料1具有很高的化学活性,并且在600–800 nm时对氢硫化物具有几乎开启的荧光响应,它对氢硫化物的选择性也比其他物种好;通过1 H NMR分析证明了检测反应的机理,并通过时变密度泛函理论(TDDFT)计算阐明了发射反应。染料1在细胞内荧光成像中的应用已成功地用于HeLa细胞的氢硫化物成像。











































 京公网安备 11010802027423号
京公网安备 11010802027423号