当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Absorptive Dissolution Testing of Supersaturating Systems: Impact of Absorptive Sink Conditions on Solution Phase Behavior and Mass Transport
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00740 Siddhi S. Hate 1 , Susan M. Reutzel-Edens 2 , Lynne S. Taylor 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00740 Siddhi S. Hate 1 , Susan M. Reutzel-Edens 2 , Lynne S. Taylor 1
Affiliation
|
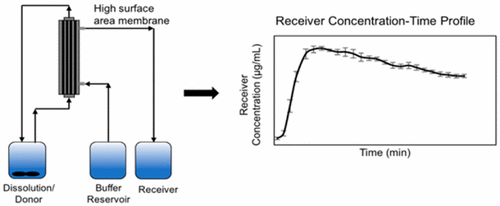
One of the most commonly used formulation development tools is dissolution testing. However, for solubility enhancing formulations, a simple closed compartment conventional dissolution apparatus operating under sink conditions often fails to predict oral bioavailability and differentiate between formulations. Hence, increasing attention is being paid to combined dissolution–absorption testing. The currently available mass transport apparatuses, however, have certain limitations, the most important being the small membrane surface area, which results in slow mass transfer. In this study, a novel high surface area, flow-through absorptive dissolution testing apparatus was developed and tested on a weakly basic model drug, nevirapine. Following optimization of the experimental parameters, the mass transfer attained for a nevirapine solution was 30 times higher in 60 min as compared to a side-by-side diffusion cell. To further evaluate the system, nevirapine powder and commercial tablets were first dissolved at an acidic pH, followed by pH increase, creating a supersaturated solution. Detailed information related to the extent of supersaturation achieved in crystallizing and noncrystallizing systems could be obtained from the combined dissolution–mass transport measurements. Differences in donor cell compartment concentration–time profiles were noted for absorptive versus closed compartment conditions. It is anticipated that this approach could be a promising tool to identify solubility enabling formulations that perform optimally in vivo.
中文翻译:

过饱和系统的吸收溶解测试:吸收沉条件对溶液相行为和传质的影响
溶出度测试是最常用的配方开发工具之一。然而,对于提高溶解度的制剂,在水槽条件下操作的简单的密闭式常规溶出装置通常无法预测口服生物利用度并无法区分制剂。因此,越来越多的关注结合溶出-吸收测试。然而,当前可用的传质设备具有一定的局限性,最重要的是较小的膜表面积,这导致传质缓慢。在这项研究中,开发了一种新型的高表面积流通吸收吸收溶出度测试设备,并在弱碱性模型药物奈韦拉平上进行了测试。优化实验参数后,与并排扩散池相比,奈韦拉平溶液在60分钟内获得的传质高出30倍。为了进一步评估该系统,首先将奈韦拉平粉剂和市售片剂在酸性pH值下溶解,然后将其pH值升高,从而形成过饱和溶液。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。奈韦拉平粉和市售片剂首先在酸性pH下溶解,然后pH升高,形成过饱和溶液。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。奈韦拉平粉和市售片剂首先在酸性pH下溶解,然后pH升高,形成过饱和溶液。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。体内。
更新日期:2017-10-19
中文翻译:

过饱和系统的吸收溶解测试:吸收沉条件对溶液相行为和传质的影响
溶出度测试是最常用的配方开发工具之一。然而,对于提高溶解度的制剂,在水槽条件下操作的简单的密闭式常规溶出装置通常无法预测口服生物利用度并无法区分制剂。因此,越来越多的关注结合溶出-吸收测试。然而,当前可用的传质设备具有一定的局限性,最重要的是较小的膜表面积,这导致传质缓慢。在这项研究中,开发了一种新型的高表面积流通吸收吸收溶出度测试设备,并在弱碱性模型药物奈韦拉平上进行了测试。优化实验参数后,与并排扩散池相比,奈韦拉平溶液在60分钟内获得的传质高出30倍。为了进一步评估该系统,首先将奈韦拉平粉剂和市售片剂在酸性pH值下溶解,然后将其pH值升高,从而形成过饱和溶液。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。奈韦拉平粉和市售片剂首先在酸性pH下溶解,然后pH升高,形成过饱和溶液。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。奈韦拉平粉和市售片剂首先在酸性pH下溶解,然后pH升高,形成过饱和溶液。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。有关结晶和非结晶系统中过饱和程度的详细信息,可从结合的溶解-质量迁移测量中获得。对于吸收性和封闭性条件,记录了供体细胞室浓度-时间曲线的差异。可以预期,这种方法可能是一种有前途的工具,可用于确定可最佳溶解性的配方。体内。



























 京公网安备 11010802027423号
京公网安备 11010802027423号