当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of highly active anti-Pneumocystis bisbenzamidines: insight into the influence of selected substituents on the in vitro activity
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-05 00:00:00 , DOI: 10.1039/c7md00445a D. Maciejewska 1, 2, 3, 4, 5 , J. Żabiński 1, 2, 3, 4, 5 , M. Rezler 1, 2, 3, 4, 5 , P. Kaźmierczak 1, 2, 3, 4, 5 , M. S. Collins 6, 7, 8, 9, 10 , L. Ficker 6, 7, 8, 9, 10 , M. T. Cushion 6, 7, 8, 9, 10
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-05 00:00:00 , DOI: 10.1039/c7md00445a D. Maciejewska 1, 2, 3, 4, 5 , J. Żabiński 1, 2, 3, 4, 5 , M. Rezler 1, 2, 3, 4, 5 , P. Kaźmierczak 1, 2, 3, 4, 5 , M. S. Collins 6, 7, 8, 9, 10 , L. Ficker 6, 7, 8, 9, 10 , M. T. Cushion 6, 7, 8, 9, 10
Affiliation
|
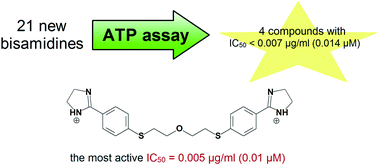
Here we describe the potency of 21 pentamidine analogues against the fungal pathogen, Pneumocystis carinii, in an ATP bioluminescent assay with toxicity profiles in 2 mammalian cell lines. Reduction of two 5-methyl-1,2,4-oxadiazole rings was applied to the synthesis of acid-labile bisamidines. Anti-Pneumocystis activity is discussed in the context of 3 groups of compounds depending on the main structural changes of the pentamidine lead structure. The groups include: 1) 1,4-bis(methylene)piperazine derivatives 1–5; 2) alkanediamide derivatives 6–10; 3) alkane-derived bisbenzamidines 11–21. IC50 values of 18 compounds were lower than the IC50 of pentamidine. Four bisamidines were active at nanogram concentrations. Introduction of sulfur atoms in the alkane bridge, replacement of the amidino groups with imidazoline rings, or attachment of nitro or amino groups to the benzene rings is responsible for remarkable activity of the new leading structures. The vast majority of compounds, including four highly active ones, can be classified as mild or nontoxic to host cells. These compounds show promise as candidates for new anti-Pneumocystis agents.
中文翻译:

高活性抗肺孢子虫双苯甲m的开发:洞悉所选取代基对体外活性的影响
在这里,我们描述了21种喷他idine类似物针对真菌病原体卡氏肺孢子虫(Pneumocystis carinii)的效力,在2种哺乳动物细胞系中具有毒性谱的ATP生物发光测定中。将两个5-甲基-1,2,4-恶二唑环的还原反应用于酸不稳定的双bis的合成。根据戊tam前导结构的主要结构变化,在3组化合物的背景下讨论了抗肺孢子虫活性。这些组包括:1)1,4-双(亚甲基)哌嗪衍生物1-5;2)链烷二酰胺衍生物6-10;3)烷烃衍生的双苯甲m 11–21。18种化合物的IC 50值低于IC 50喷他idine。四个双am在纳克浓度下具有活性。在烷烃桥中引入硫原子,用咪唑啉环取代a基,或将硝基或氨基与苯环连接,是导致新的前导结构显着活性的原因。绝大多数化合物,包括四种高活性化合物,可被分类为对宿主细胞温和或无毒。这些化合物显示出有望用作新的抗肺气肿药的候选药物。
更新日期:2017-10-18
中文翻译:

高活性抗肺孢子虫双苯甲m的开发:洞悉所选取代基对体外活性的影响
在这里,我们描述了21种喷他idine类似物针对真菌病原体卡氏肺孢子虫(Pneumocystis carinii)的效力,在2种哺乳动物细胞系中具有毒性谱的ATP生物发光测定中。将两个5-甲基-1,2,4-恶二唑环的还原反应用于酸不稳定的双bis的合成。根据戊tam前导结构的主要结构变化,在3组化合物的背景下讨论了抗肺孢子虫活性。这些组包括:1)1,4-双(亚甲基)哌嗪衍生物1-5;2)链烷二酰胺衍生物6-10;3)烷烃衍生的双苯甲m 11–21。18种化合物的IC 50值低于IC 50喷他idine。四个双am在纳克浓度下具有活性。在烷烃桥中引入硫原子,用咪唑啉环取代a基,或将硝基或氨基与苯环连接,是导致新的前导结构显着活性的原因。绝大多数化合物,包括四种高活性化合物,可被分类为对宿主细胞温和或无毒。这些化合物显示出有望用作新的抗肺气肿药的候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号