当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An agent for optical imaging of TrkC-expressing, breast cancer
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1039/c7md00328e Anyanee Kamkaew 1, 2, 3, 4, 5 , Feng Li 3, 6, 7, 8 , Zheng Li 3, 6, 7, 8 , Kevin Burgess 1, 2, 3
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-09-14 00:00:00 , DOI: 10.1039/c7md00328e Anyanee Kamkaew 1, 2, 3, 4, 5 , Feng Li 3, 6, 7, 8 , Zheng Li 3, 6, 7, 8 , Kevin Burgess 1, 2, 3
Affiliation
|
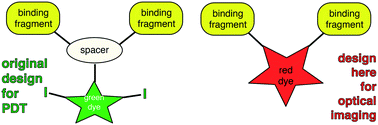
Tropomyosin receptor kinases receptor C is expressed at high levels on the surface of tumors from metastatic breast cancer, metastatic melanoma, glioblastoma, and neuroblastoma. Previous studies have shown synthetic TrkC ligands bearing agents for photodynamic therapy could be used to completely ablate 4T1 metastatic breast tumors and suppress metastatic spread in vivo. Modification of these probes (A in the text) to make them suitable for near infrared optical imaging in vivo would require a substantial increase in molecular mass (and hence increased vulnerability to undesirable absorption, metabolism and immunogenicity effects), or significant changes to the probe design which might compromise binding to TrkC in histochemical studies and on live cells. The research featured here was undertaken to investigate if the second strategy could be achieved without compromising binding to TrkC-expressing tissues. Specifically, an “aza-BODIPY” probe was synthesized to replace a spacer fragment in the original probe A. In the event, this new probe design (1a in the text) binds TrkC+ breast cancer in live cell cultures, in histochemical studies and in an in vivo murine model. Probe 1a binds TrkC+ tissues with good contrast with respect to healthy tissues, and much more strongly than an isomeric, non-TrkC binding, probe (1b) prepared as a negative control.
中文翻译:

用于表达TrkC的乳腺癌的光学成像试剂
Tropomyosin受体激酶受体C在转移性乳腺癌,转移性黑色素瘤,胶质母细胞瘤和神经母细胞瘤的肿瘤表面高表达。先前的研究表明,用于光动力疗法的合成TrkC配体轴承试剂可用于完全消融4T1转移性乳腺肿瘤并抑制体内转移性扩散。修改这些探针(本文中的A)以使其适合于体内近红外光学成像这将需要大量增加分子质量(从而增加对不良吸收,代谢和免疫原性影响的脆弱性),或对探针设计进行重大改变,这可能会损害在组织化学研究和活细胞中与TrkC的结合。此处进行的研究旨在调查第二种策略是否可以在不损害与表达TrkC的组织的结合的情况下实现。具体地,合成“氮杂-BODIPY”探针以替换原始探针A中的间隔片段。最终,这种新的探针设计(本文中的1a)可在活细胞培养,组织化学研究和体内鼠模型中结合TrkC +乳腺癌。探针1a结合TrkC +组织相对于健康组织具有良好的对比度,并且比作为阴性对照的同分异构的,非TrkC结合的探针(1b)强得多。
更新日期:2017-10-18
中文翻译:

用于表达TrkC的乳腺癌的光学成像试剂
Tropomyosin受体激酶受体C在转移性乳腺癌,转移性黑色素瘤,胶质母细胞瘤和神经母细胞瘤的肿瘤表面高表达。先前的研究表明,用于光动力疗法的合成TrkC配体轴承试剂可用于完全消融4T1转移性乳腺肿瘤并抑制体内转移性扩散。修改这些探针(本文中的A)以使其适合于体内近红外光学成像这将需要大量增加分子质量(从而增加对不良吸收,代谢和免疫原性影响的脆弱性),或对探针设计进行重大改变,这可能会损害在组织化学研究和活细胞中与TrkC的结合。此处进行的研究旨在调查第二种策略是否可以在不损害与表达TrkC的组织的结合的情况下实现。具体地,合成“氮杂-BODIPY”探针以替换原始探针A中的间隔片段。最终,这种新的探针设计(本文中的1a)可在活细胞培养,组织化学研究和体内鼠模型中结合TrkC +乳腺癌。探针1a结合TrkC +组织相对于健康组织具有良好的对比度,并且比作为阴性对照的同分异构的,非TrkC结合的探针(1b)强得多。











































 京公网安备 11010802027423号
京公网安备 11010802027423号