当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Emerging investigator series: As(V) in magnetite: incorporation and redistribution
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1039/c7em00237h Brittany L. Huhmann 1, 2, 3, 4 , Anke Neumann 5, 6, 7, 8 , Maxim I. Boyanov 4, 9, 10, 11, 12 , Kenneth M. Kemner 4, 9, 10, 11 , Michelle M. Scherer 1, 2, 3, 4
Environmental Science: Processes & Impacts ( IF 5.5 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1039/c7em00237h Brittany L. Huhmann 1, 2, 3, 4 , Anke Neumann 5, 6, 7, 8 , Maxim I. Boyanov 4, 9, 10, 11, 12 , Kenneth M. Kemner 4, 9, 10, 11 , Michelle M. Scherer 1, 2, 3, 4
Affiliation
|
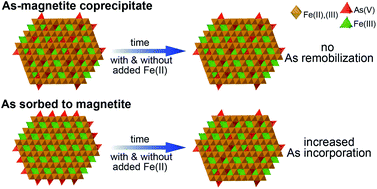
Exposure to As in groundwater negatively impacts millions of people around the globe, and As mobility in groundwater is often controlled by Fe mineral dissolution and precipitation. Additionally, trace elements can be released from and incorporated into the structure of Fe oxides in the presence of dissolved Fe(II). The potential for As to redistribute between sorbed on the magnetite surface and incorporated in the magnetite structure, however, remains unclear. In this study, we use selective chemical extraction and X-ray absorption spectroscopy (XAS) to distinguish magnetite-sorbed and incorporated As(V) and to provide evidence for As(V) incorporation during magnetite precipitation. While As in the As-magnetite coprecipitates did not redistribute between sorbed and incorporated over a 4 month period, a small, but measurable increase in incorporated As(V) of up to 13% was observed for sorbed As(V). We suggest that Fe(II)-catalyzed recrystallization of magnetite did not significantly influence the redistribution of sorbed As(V) because the extent of Fe atom exchange was small (∼10%). In addition, the extent of As redistribution was the same in the absence and presence of added aqueous Fe(II), suggesting that aqueous Fe(II) had, overall, a minor effect on As redistribution for both coprecipitated and sorbed As(V). Our results suggest that coprecipitation of As(V) with magnetite and redistribution of As(V) sorbed on magnetite are potential pathways for irreversible As(V) uptake and sequestration. These pathways are likely to play a significant role in controlling As mobility in natural systems, during human-induced redox cycling of groundwater such as aquifer storage and recovery, as well as in iron oxide-based As removal systems.
中文翻译:

新兴研究者系列:磁铁矿中的As(V):结合和再分布
地下水中As的暴露会对全球数以百万计的人造成负面影响,而As在地下水中的流动性通常受Fe矿物溶解和沉淀的控制。另外,在溶解的Fe(II)的存在下,微量元素可以从Fe氧化物中释放出来并掺入其中。但是,尚不清楚As在磁铁矿表面吸附和掺入磁铁矿结构之间重新分布的可能性。在这项研究中,我们使用选择性化学萃取和X射线吸收光谱(XAS)来区分磁铁矿吸附和结合的As(V)并为As(V)磁铁矿沉淀过程中的结合。尽管砷在磁铁矿中的共沉淀物在4个月内未在吸附和结合之间重新分布,但对于吸附的As(V),观察到的As(V)的结合量很小但可测量的增加,最高可达13%。我们认为,Fe(II)催化的磁铁矿重结晶不会显着影响吸附的As(V)的再分布,因为Fe原子交换的程度很小(〜10%)。此外,在不存在和存在Fe(II)水溶液的情况下,As的重新分布程度相同,这表明,对于共沉淀和吸附的As(),Fe(II)总体上对As的重新分布影响较小。V)。我们的结果表明,As(V)与磁铁矿的共沉淀和吸附在磁铁矿上的As(V)的重新分布是不可逆的As(V)吸收和螯合的潜在途径。这些途径可能在控制人为引起的地下水的氧化还原循环(如含水层的存储和回收)以及基于氧化铁的As去除系统中的自然系统中,在控制As迁移中起重要作用。
更新日期:2017-10-18
中文翻译:

新兴研究者系列:磁铁矿中的As(V):结合和再分布
地下水中As的暴露会对全球数以百万计的人造成负面影响,而As在地下水中的流动性通常受Fe矿物溶解和沉淀的控制。另外,在溶解的Fe(II)的存在下,微量元素可以从Fe氧化物中释放出来并掺入其中。但是,尚不清楚As在磁铁矿表面吸附和掺入磁铁矿结构之间重新分布的可能性。在这项研究中,我们使用选择性化学萃取和X射线吸收光谱(XAS)来区分磁铁矿吸附和结合的As(V)并为As(V)磁铁矿沉淀过程中的结合。尽管砷在磁铁矿中的共沉淀物在4个月内未在吸附和结合之间重新分布,但对于吸附的As(V),观察到的As(V)的结合量很小但可测量的增加,最高可达13%。我们认为,Fe(II)催化的磁铁矿重结晶不会显着影响吸附的As(V)的再分布,因为Fe原子交换的程度很小(〜10%)。此外,在不存在和存在Fe(II)水溶液的情况下,As的重新分布程度相同,这表明,对于共沉淀和吸附的As(),Fe(II)总体上对As的重新分布影响较小。V)。我们的结果表明,As(V)与磁铁矿的共沉淀和吸附在磁铁矿上的As(V)的重新分布是不可逆的As(V)吸收和螯合的潜在途径。这些途径可能在控制人为引起的地下水的氧化还原循环(如含水层的存储和回收)以及基于氧化铁的As去除系统中的自然系统中,在控制As迁移中起重要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号