Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2017-10-18 , DOI: 10.1016/j.apcatb.2017.10.037 Junsong Guo , Rongrong Chen , Fu-Chun Zhu , Shi-Gang Sun , Hebe M. Villullas
|
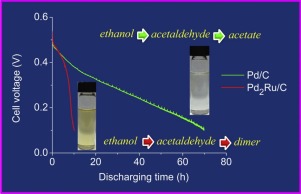
Ethanol oxidation reaction (EOR) on Pd2Ru/C and Pd/C catalysts in alkaline media is studied comprehensively by cyclic voltammetry, chronoamperometry, in situ FTIR, single fuel cell test and electrochemical impedance spectroscopy measurements. The results show that, as compared to Pd/C, Pd2Ru/C favors acetaldehyde formation and hinders its oxidation. Based on X-ray absorption data, which evidence that Ru promotes a larger electronic vacancy of the Pd 4d band, it is expected that the formation of adsorbed ethoxy is favored on Pd2Ru/C and followed by its oxidation to acetaldehyde facilitated by oxygenated species provided by Ru. In contrast, acetaldehyde oxidation is more difficult on Pd2Ru/C than on Pd/C likely because the adsorption energy of the reactive species is increased. We also show that the performance of Pd2Ru/C anode in alkaline direct ethanol fuel cell (ADEFC) is initially better but degrades much more rapidly than that with Pd/C anode under the same test conditions. The degradation is demonstrated to result from the accumulation of large amounts of acetaldehyde, which in alkaline media forms dimers by the aldol condensation reaction. The dimers tend to be responsible for blocking the active sites for further ethanol oxidation. This comprehensive study provides new understandings of the roles of Ru in Pd2Ru/C for EOR in alkaline media, unveils the causes of the performance degradation of fuel cells with Pd2Ru/C and demonstrates that initial good performances are not necessarily a valid criterion for selecting appropriate anode catalysts for ADEFC applications.
中文翻译:

对碱性直接乙醇燃料电池中Pd / C和Pd 2 Ru / C催化剂上乙醇氧化反应机理的新认识
通过循环伏安法,计时电流法,原位FTIR,单燃料电池测试和电化学阻抗谱测量等方法,对碱性介质中Pd 2 Ru / C和Pd / C催化剂上的乙醇氧化反应(EOR)进行了综合研究。结果表明,与Pd / C相比,Pd 2 Ru / C有利于乙醛形成并阻碍其氧化。根据X射线吸收数据,该证据表明Ru促进了Pd 4d谱带的更大电子空位,预期在Pd 2 Ru / C上有利于形成吸附的乙氧基,然后通过氧化将其氧化为乙醛。茹提供的物种。相比之下,乙醛在Pd 2上的氧化更困难Ru / C比在Pd / C上高可能是因为反应性物质的吸附能增加了。我们还表明,在相同的测试条件下,Pd 2 Ru / C阳极在碱性直接乙醇燃料电池(ADEFC)中的性能最初要好一些,但降解速度要比Pd / C阳极快得多。已证明降解是由于大量乙醛的积累引起的,该乙醛在碱性介质中通过醛醇缩合反应形成二聚体。二聚体倾向于负责封闭用于进一步乙醇氧化的活性位点。这项全面的研究提供了对Ru在Pd 2中的作用的新认识,Ru / C对于碱性介质中的EOR,揭示了Pd 2导致燃料电池性能下降的原因。Ru / C并证明初始良好性能不一定是为ADEFC应用选择合适的阳极催化剂的有效标准。



























 京公网安备 11010802027423号
京公网安备 11010802027423号