当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Cancer Therapy Using Fusion Protein of TNFα and Tumor-Associated Fibronectin-Specific Aptide
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-10-11 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00520 Hyungsu Jeon , Daejin Kim , Minsuk Choi , Sukmo Kang , Jin Yong Kim , Sunghyun Kim 1 , Sangyong Jon
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-10-11 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00520 Hyungsu Jeon , Daejin Kim , Minsuk Choi , Sukmo Kang , Jin Yong Kim , Sunghyun Kim 1 , Sangyong Jon
Affiliation
|
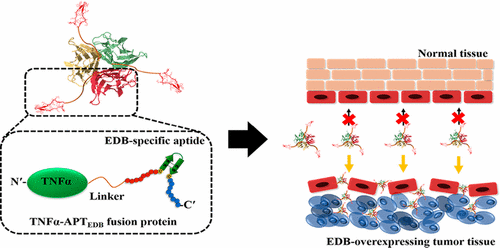
Tumor necrosis factor-α has shown potent antitumor effects in preclinical and clinical studies. However, severe side effects at less than therapeutic doses have limited its systemic delivery, prompting the need for a new strategy for targeted delivery of the protein to tumors. Here, we report a fusion protein of mouse tumor necrosis factor (TNF)-α (mTNFα) and a cancer-targeting, high-affinity aptide and investigate its therapeutic efficacy in tumor-bearing mice. A fusion protein consisting of mTNFα, a linker, and an aptide specific to extra domain B (EDB) of fibronectin (APTEDB), designated mTNFα-APTEDB, was successfully produced by expression in Escherichia coli. mTNFα-APTEDB retained specificity and affinity for its target, EDB. In mice bearing EDB-overexpressing fibrosarcomas, mTNFα-APTEDB showed greater efficacy in inhibiting tumor growth than mTNFα alone or mTNFα linked to a nonrelevant aptide, without causing an appreciable loss in body weight. Moreover, in vivo antitumor efficacy was further significantly increased by combination treatment with the chemotherapeutic drug, melphalan, suggesting a synergistic effect attributable to enhanced drug uptake into the tumor as a result of TNFα-mediated enhanced vascular permeability. These results suggest that a fusion protein of mTNFα with a cancer-targeting peptide could be a new anticancer therapeutic option for ensuring potent antitumor efficacy after systemic delivery.
中文翻译:

使用TNFα和肿瘤相关纤连蛋白特异性肽融合蛋白的靶向癌症治疗
在临床前和临床研究中,肿瘤坏死因子-α已显示出有效的抗肿瘤作用。然而,低于治疗剂量的严重副作用限制了其全身递送,从而引发了针对将蛋白质靶向递送至肿瘤的新策略的需求。在这里,我们报告小鼠肿瘤坏死因子(TNF)-α(mTNFα)和靶向癌症的高亲和力适肽的融合蛋白,并研究其在荷瘤小鼠中的治疗功效。由mTNFα,接头的融合蛋白和纤连蛋白的一个特定aptide额外结构域B(EDB)(APT EDB),指定mTNFα-APT EDB,成功地通过在表达产生大肠杆菌。mTNFα,APT EDB保留了针对其目标EDB的特异性和亲和力。在小鼠中,轴承EDB过量表达的纤维肉瘤,mTNFα-APT EDB表现出更大的功效在单独抑制肿瘤生长超过mTNFα或mTNFα连接于非相关aptide,而不引起体重的明显损失。此外,通过与化学治疗药物美法仑的联合治疗,体内抗肿瘤功效进一步显着提高,表明由于TNFα介导的增强的血管渗透性,可归因于药物对肿瘤的吸收增加而产生的协同效应。这些结果表明,mTNFα与癌症靶向肽的融合蛋白可能是一种新的抗癌治疗选择,可确保在全身分娩后有效的抗肿瘤功效。
更新日期:2017-10-12
中文翻译:

使用TNFα和肿瘤相关纤连蛋白特异性肽融合蛋白的靶向癌症治疗
在临床前和临床研究中,肿瘤坏死因子-α已显示出有效的抗肿瘤作用。然而,低于治疗剂量的严重副作用限制了其全身递送,从而引发了针对将蛋白质靶向递送至肿瘤的新策略的需求。在这里,我们报告小鼠肿瘤坏死因子(TNF)-α(mTNFα)和靶向癌症的高亲和力适肽的融合蛋白,并研究其在荷瘤小鼠中的治疗功效。由mTNFα,接头的融合蛋白和纤连蛋白的一个特定aptide额外结构域B(EDB)(APT EDB),指定mTNFα-APT EDB,成功地通过在表达产生大肠杆菌。mTNFα,APT EDB保留了针对其目标EDB的特异性和亲和力。在小鼠中,轴承EDB过量表达的纤维肉瘤,mTNFα-APT EDB表现出更大的功效在单独抑制肿瘤生长超过mTNFα或mTNFα连接于非相关aptide,而不引起体重的明显损失。此外,通过与化学治疗药物美法仑的联合治疗,体内抗肿瘤功效进一步显着提高,表明由于TNFα介导的增强的血管渗透性,可归因于药物对肿瘤的吸收增加而产生的协同效应。这些结果表明,mTNFα与癌症靶向肽的融合蛋白可能是一种新的抗癌治疗选择,可确保在全身分娩后有效的抗肿瘤功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号