当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Orthoester functionalized N-guanidino derivatives of 1,5-dideoxy-1,5-imino-D-xylitol as pH-responsive inhibitors of β-glucocerebrosidase
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-10 00:00:00 , DOI: 10.1039/c7md00480j Alen Sevšek 1, 2, 3, 4 , Javier Sastre Toraño 1, 2, 3, 4 , Linda Quarles van Ufford 1, 2, 3, 4 , Ed E. Moret 1, 2, 3, 4 , Roland J. Pieters 1, 2, 3, 4 , Nathaniel I. Martin 1, 2, 3, 4
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-10 00:00:00 , DOI: 10.1039/c7md00480j Alen Sevšek 1, 2, 3, 4 , Javier Sastre Toraño 1, 2, 3, 4 , Linda Quarles van Ufford 1, 2, 3, 4 , Ed E. Moret 1, 2, 3, 4 , Roland J. Pieters 1, 2, 3, 4 , Nathaniel I. Martin 1, 2, 3, 4
Affiliation
|
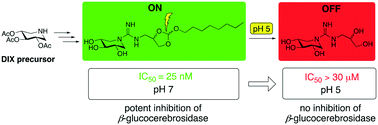
Alkylated guanidino derivatives of 1,5-dideoxy-1,5-imino-D-xylitol bearing an orthoester moiety were prepared using a concise synthetic protocol. Inhibition assays with a panel of glycosidases revealed that one of the compounds prepared displays potent inhibition against human β-glucocerebrosidase (GBA) at pH 7.0 with IC50 values in the low nanomolar range. Notably, a significant drop in inhibitory activity is observed when the same compound is tested at pH 5.2. This pH sensitive activity is due to degradation of the orthoester functionality at lower pH accompanied by loss of the alkyl group. This approach provides a degree of control in tuning enzyme inhibition based on the local pH. Compounds like those here described may serve as tools for studying various lysosomal storage disorders such as Gaucher disease. In this regard, the most active compound was also evaluated as a potential pharmacological chaperone by assessing its effect on GBA activity in an assay employing fibroblasts from Gaucher patients.
中文翻译:

1,5-二脱氧-1,5-亚氨基-D-木糖醇的原酸酯官能化N-胍基衍生物作为β-葡萄糖脑苷脂酶的pH响应抑制剂
使用简洁的合成方案制备带有原酸酯部分的1,5-二脱氧-1,5-亚氨基-D-木糖醇的烷基化胍基衍生物。用一组糖苷酶进行的抑制分析表明,所制备的化合物之一在pH 7.0的条件下具有IC 50对人β-葡萄糖脑苷脂酶(GBA)的有效抑制作用值在低纳摩尔范围内。值得注意的是,当在pH 5.2下测试相同化合物时,观察到抑制活性显着下降。这种对pH敏感的活性是由于在较低pH下原酸酯官能团的降解并伴有烷基的丢失。该方法基于局部pH在调节酶抑制方面提供了一定程度的控制。如本文所述的那些化合物可以用作研究各种溶酶体贮积病例如高雪氏病的工具。在这方面,通过在使用来自Gaucher患者的成纤维细胞的测定中评估其对GBA活性的作用,还评价了最具活性的化合物作为潜在的药理伴侣。
更新日期:2017-10-12
中文翻译:

1,5-二脱氧-1,5-亚氨基-D-木糖醇的原酸酯官能化N-胍基衍生物作为β-葡萄糖脑苷脂酶的pH响应抑制剂
使用简洁的合成方案制备带有原酸酯部分的1,5-二脱氧-1,5-亚氨基-D-木糖醇的烷基化胍基衍生物。用一组糖苷酶进行的抑制分析表明,所制备的化合物之一在pH 7.0的条件下具有IC 50对人β-葡萄糖脑苷脂酶(GBA)的有效抑制作用值在低纳摩尔范围内。值得注意的是,当在pH 5.2下测试相同化合物时,观察到抑制活性显着下降。这种对pH敏感的活性是由于在较低pH下原酸酯官能团的降解并伴有烷基的丢失。该方法基于局部pH在调节酶抑制方面提供了一定程度的控制。如本文所述的那些化合物可以用作研究各种溶酶体贮积病例如高雪氏病的工具。在这方面,通过在使用来自Gaucher患者的成纤维细胞的测定中评估其对GBA活性的作用,还评价了最具活性的化合物作为潜在的药理伴侣。



























 京公网安备 11010802027423号
京公网安备 11010802027423号