当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of the Selectivity of GenN, an Aminoglycoside N-Methyltransferase Involved in Gentamicin Biosynthesis
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acschembio.7b00466 Priscila dos Santos Bury 1 , Fanglu Huang 2 , Sicong Li 3 , Yuhui Sun 3 , Peter F. Leadlay 2 , Marcio Vinicius Bertacine Dias 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acschembio.7b00466 Priscila dos Santos Bury 1 , Fanglu Huang 2 , Sicong Li 3 , Yuhui Sun 3 , Peter F. Leadlay 2 , Marcio Vinicius Bertacine Dias 1
Affiliation
|
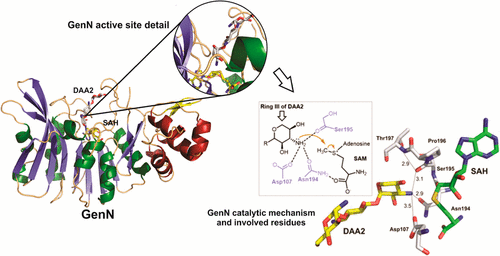
Gentamicins are heavily methylated, clinically valuable pseudotrisaccharide antibiotics produced by Micromonospora echinospora. GenN has been characterized as an S-adenosyl-l-methionine-dependent methyltransferase with low sequence similarity to other enzymes. It is responsible for the 3″-N-methylation of 3″-dehydro-3″-amino-gentamicin A2, an essential modification of ring III in the biosynthetic pathway to the gentamicin C complex. Purified recombinant GenN also efficiently catalyzes 3″-N-methylation of related aminoglycosides kanamycin B and tobramycin, which both contain an additional hydroxymethyl group at the C5″ position in ring III. We have obtained eight cocrystal structures of GenN, at a resolution of 2.2 Å or better, including the binary complex of GenN and S-adenosyl-l-homocysteine (SAH) and the ternary complexes of GenN, SAH, and several aminoglycosides. The GenN structure reveals several features not observed in any other N-methyltransferase that fit it for its role in gentamicin biosynthesis. These include a novel N-terminal domain that might be involved in protein:protein interaction with upstream enzymes of the gentamicin X2 biosynthesis and two long loops that are involved in aminoglycoside substrate recognition. In addition, the analysis of structures of GenN in complex with different ligands, supported by the results of active site mutagenesis, has allowed us to propose a catalytic mechanism and has revealed the structural basis for the surprising ability of native GenN to act on these alternative substrates.
中文翻译:

庆大霉素生物合成中氨基糖苷N-甲基转移酶GenN选择性的结构基础
庆大霉素是由Micromonospora echinospora生产的具有高度甲基化的,临床上有价值的假三糖抗生素。GenN已经被表征为与其他酶具有低序列相似性的S-腺苷-1-甲硫氨酸依赖性甲基转移酶。它负责3″-脱氢-3″-氨基-庆大霉素A2的3″ -N-甲基化,这是对庆大霉素C复合物的生物合成途径中环III的必要修饰。纯化的重组GenN还可以有效催化3''- N相关的氨基糖苷卡那霉素B和妥布霉素的甲基化,它们在环III的C5''位置都含有一个额外的羟甲基。我们获得了分辨率为2.2或更佳的GenN的八个共晶结构,包括GenN和S-腺苷-1-同型半胱氨酸(SAH)的二元复合物以及GenN,SAH的三元复合物和几种氨基糖苷。GenN结构揭示了其他N-甲基转移酶适合其在庆大霉素生物合成中的作用。这些包括可能与蛋白质:蛋白质与庆大霉素X2生物合成上游酶的相互作用有关的新的N末端结构域,以及两个与氨基糖苷底物识别有关的长环。此外,由活性位点诱变的结果支持的具有不同配体的复杂GenN结构的分析,使我们提出了一种催化机制,并揭示了天然GenN出人意料地作用于这些替代物的能力的结构基础。基材。
更新日期:2017-10-09
中文翻译:

庆大霉素生物合成中氨基糖苷N-甲基转移酶GenN选择性的结构基础
庆大霉素是由Micromonospora echinospora生产的具有高度甲基化的,临床上有价值的假三糖抗生素。GenN已经被表征为与其他酶具有低序列相似性的S-腺苷-1-甲硫氨酸依赖性甲基转移酶。它负责3″-脱氢-3″-氨基-庆大霉素A2的3″ -N-甲基化,这是对庆大霉素C复合物的生物合成途径中环III的必要修饰。纯化的重组GenN还可以有效催化3''- N相关的氨基糖苷卡那霉素B和妥布霉素的甲基化,它们在环III的C5''位置都含有一个额外的羟甲基。我们获得了分辨率为2.2或更佳的GenN的八个共晶结构,包括GenN和S-腺苷-1-同型半胱氨酸(SAH)的二元复合物以及GenN,SAH的三元复合物和几种氨基糖苷。GenN结构揭示了其他N-甲基转移酶适合其在庆大霉素生物合成中的作用。这些包括可能与蛋白质:蛋白质与庆大霉素X2生物合成上游酶的相互作用有关的新的N末端结构域,以及两个与氨基糖苷底物识别有关的长环。此外,由活性位点诱变的结果支持的具有不同配体的复杂GenN结构的分析,使我们提出了一种催化机制,并揭示了天然GenN出人意料地作用于这些替代物的能力的结构基础。基材。










































 京公网安备 11010802027423号
京公网安备 11010802027423号