PLOS Medicine ( IF 10.5 ) Pub Date : 2017-10-06 , DOI: 10.1371/journal.pmed.1002402 Selidji T Agnandji 1, 2, 3 , José F Fernandes 1, 2 , Emmanuel B Bache 1 , Régis M Obiang Mba 1 , Jessica S Brosnahan 1, 2, 3 , Lumeka Kabwende 1 , Paul Pitzinger 1, 2, 4 , Pieter Staarink 1, 5 , Marguerite Massinga-Loembe 1 , Verena Krähling 3, 6 , Nadine Biedenkopf 6 , Sarah Katharina Fehling 6 , Thomas Strecker 6 , David J Clark 7 , Henry M Staines 7 , Jay W Hooper 8 , Peter Silvera 8 , Vasee Moorthy 9 , Marie-Paule Kieny 9 , Akim A Adegnika 1, 2, 3, 10 , Martin P Grobusch 1, 2, 5 , Stephan Becker 3, 6 , Michael Ramharter 1, 2, 4 , Benjamin Mordmüller 1, 2, 3 , Bertrand Lell 1, 2, 3 , , Sanjeev Krishna 1, 2, 7, 11 , Peter G Kremsner 1, 2, 3
|
Background
The rVSVΔG-ZEBOV-GP vaccine prevented Ebola virus disease when used at 2 × 107 plaque-forming units (PFU) in a trial in Guinea. This study provides further safety and immunogenicity data.
Methods and findings
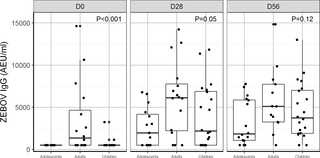
A randomised, open-label phase I trial in Lambaréné, Gabon, studied 5 single intramuscular vaccine doses of 3 × 103, 3 × 104, 3 × 105, 3 × 106, or 2 × 107 PFU in 115 adults and a dose of 2 × 107 PFU in 20 adolescents and 20 children. The primary objective was safety and tolerability 28 days post-injection. Immunogenicity, viraemia, and shedding post-vaccination were evaluated as secondary objectives. In adults, mild-to-moderate adverse events were frequent, but there were no serious or severe adverse events related to vaccination. Before vaccination, Zaire Ebola virus (ZEBOV)–glycoprotein (GP)–specific and ZEBOV antibodies were detected in 11% and 27% of adults, respectively. In adults, 74%–100% of individuals who received a dose 3 × 104, 3 × 105, 3 × 106, or 2 × 107 PFU had a ≥4.0-fold increase in geometric mean titres (GMTs) of ZEBOV-GP-specific antibodies at day 28, reaching GMTs of 489 (95% CI: 264–908), 556 (95% CI: 280–1,101), 1,245 (95% CI: 899–1,724), and 1,503 (95% CI: 931–2,426), respectively. Twenty-two percent of adults had a ≥4-fold increase of ZEBOV antibodies, with GMTs at day 28 of 1,015 (647–1,591), 1,887 (1,154–3,085), 1,445 (1,013–2,062), and 3,958 (2,249–6,967) for the same doses, respectively. These antibodies persisted up to day 180 for doses ≥3 × 105 PFU. Adults with antibodies before vaccination had higher GMTs throughout. Neutralising antibodies were detected in more than 50% of participants at doses ≥3 × 105 PFU. As in adults, no serious or severe adverse events related to vaccine occurred in adolescents or children. At day 2, vaccine RNA titres were higher for adolescents and children than adults. At day 7, 78% of adolescents and 35% of children had recombinant vesicular stomatitis virus RNA detectable in saliva. The vaccine induced high GMTs of ZEBOV-GP-specific antibodies at day 28 in adolescents, 1,428 (95% CI: 1,025–1,989), and children, 1,620 (95% CI: 806–3,259), and in both groups antibody titres increased up to day 180. The absence of a control group, lack of stratification for baseline antibody status, and imbalances in male/female ratio are the main limitations of this study.
Conclusions
Our data confirm the acceptable safety and immunogenicity profile of the 2 × 107 PFU dose in adults and support consideration of lower doses for paediatric populations and those who request boosting.
Trial registration
Pan African Clinical Trials Registry PACTR201411000919191
中文翻译:

rVSVΔG-ZEBOV-GP 埃博拉疫苗在加蓬兰巴雷内成人和儿童中的安全性和免疫原性:I 期随机试验
背景
在几内亚的一项试验中,rVSV Δ G-ZEBOV-GP 疫苗以 2 × 10 7噬菌斑形成单位 (PFU)使用时可预防埃博拉病毒病。该研究提供了进一步的安全性和免疫原性数据。
方法和发现
在加蓬兰巴雷内进行的一项随机、开放标签的 I 期试验在 115 名成人中研究了 5 个单次肌肉注射疫苗,分别为 3 × 10 3、3 × 10 4、3 × 10 5、3 × 10 6或 2 × 10 7 PFU和 2 × 10 7的剂量20 名青少年和 20 名儿童的 PFU。主要目标是注射后 28 天的安全性和耐受性。免疫原性、病毒血症和疫苗接种后脱落被评估为次要目标。在成人中,轻度至中度不良事件很常见,但没有与疫苗接种相关的严重或严重不良事件。在接种疫苗之前,分别在 11% 和 27% 的成年人中检测到扎伊尔埃博拉病毒 (ZEBOV)-糖蛋白 (GP) 特异性和 ZEBOV 抗体。在成人中,74%–100% 的个体接受了 3 × 10 4、3 × 10 5、3 × 10 6或 2 × 10 7的剂量在第 28 天,PFU 的 ZEBOV-GP 特异性抗体的几何平均滴度 (GMTs) 增加了 ≥4.0 倍,达到 489 (95% CI: 264–908)、556 (95% CI: 280–1,101) 的 GMTs 、1,245 (95% CI: 899–1,724) 和 1,503 (95% CI: 931–2,426)。22% 的成年人的 ZEBOV 抗体增加 ≥ 4 倍,GMT 在第 28 天的 1,015 (647–1,591)、1,887 (1,154–3,085)、1,445 (1,013–2,062) 和 3,958 (2,249–6,967) ) 分别用于相同的剂量。对于≥3 × 10 5 PFU的剂量,这些抗体持续到第 180 天。接种疫苗前有抗体的成年人自始至终都有较高的 GMT。超过 50% 的参与者在剂量 ≥3 × 10 5时检测到中和抗体PFU。与成人一样,青少年或儿童未发生与疫苗相关的严重或严重不良事件。在第 2 天,青少年和儿童的疫苗 RNA 滴度高于成人。在第 7 天,78% 的青少年和 35% 的儿童在唾液中检测到重组水泡性口炎病毒 RNA。该疫苗在第 28 天在青少年 1,428(95% CI:1,025-1,989)和儿童(1,620(95% CI:806-3,259))中诱导了 ZEBOV-GP 特异性抗体的高 GMT,并且两组的抗体滴度均增加直到第 180 天。缺乏对照组、缺乏对基线抗体状态的分层以及男性/女性比例的不平衡是本研究的主要局限性。
结论
我们的数据证实了 2 × 10 7 PFU 剂量在成人中可接受的安全性和免疫原性特征,并支持对儿科人群和需要加强剂量的人群考虑较低剂量。
试用注册
泛非临床试验注册PACTR201411000919191











































 京公网安备 11010802027423号
京公网安备 11010802027423号