当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper Sensing in Alkaline Electrolyte Using Anodic Stripping Voltammetry by Means of a Lead Mediator
Electroanalysis ( IF 2.7 ) Pub Date : 2017-10-05 , DOI: 10.1002/elan.201700526 Jonathon Duay 1 , Joed E. Ortiz-Santiago 1 , Timothy N. Lambert 1
Electroanalysis ( IF 2.7 ) Pub Date : 2017-10-05 , DOI: 10.1002/elan.201700526 Jonathon Duay 1 , Joed E. Ortiz-Santiago 1 , Timothy N. Lambert 1
Affiliation
|
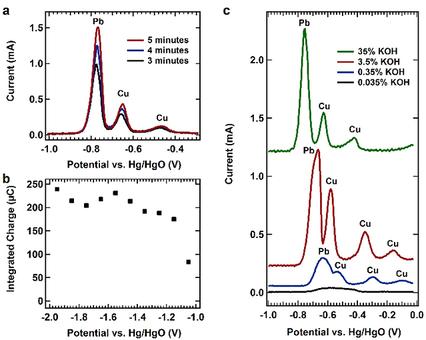
Anodic stripping voltammetry (ASV) is an analysis technique that permits the selective and quantitative analysis of metal ion species in solution. It is most commonly applied in neutral to acidic electrolyte largely due to inherent metal ion solubility. Bismuth (Bi) is a common film used for ASV due to its good sensitivity, overall stability and insensitivity to O2. ASV, utilizing a Bi film, along with cadmium (Cd) and lead (Pb) as the plating mediators, has recently been adapted to determine zinc (Zn) concentrations in highly alkaline environments (30 % NaOH or 35 % M KOH). Successful analysis of Zn in alkaline relies on the ability of the hydroxide to form soluble metal anion species, such as Bi(OH)4− and Zn(OH)42−. Here, we look to extend this technique to detect and quantify copper (Cu) ions in these highly basic electrolytes. However, in general, the use of ASV to detect and quantify Cu ion concentrations is notoriously difficult as the Cu stripping peak potential overlays with that of Bi from the common Bi film electrode. Here, an ASV method for determining Cu concentration in alkaline solutions is developed utilizing Pb as a deposition mediator. As such, it was found that when analyzing Cu solutions in the presence of Pb, the stripping voltammetry curves present separate and defined Cu stripping peaks. Different analyzes were made to find the best stripping voltammetry performance conditions. As such, an accumulation time of 5 minutes, an accumulation potential of≤−1.45 V vs. Hg/HgO, and a concentration of 35 wt% KOH were determined to be the conditions that presented the best ASV results. Utilizing these conditions, calibration curves in the presence of 5.0 ppm Pb showed the best linear stripping signal correlation with an r-squared value of 0.991 and a limit of detection (LOD) of 0.67 ppm. These results give way to evaluating Cu concentrations using ASV in aqueous alkaline solutions.
中文翻译:

通过铅介体使用阳极溶出伏安法检测碱性电解质中的铜
阳极溶出伏安法 (ASV) 是一种分析技术,可以对溶液中的金属离子种类进行选择性和定量分析。由于固有的金属离子溶解度,它最常用于中性至酸性电解质中。铋 (Bi) 是用于 ASV 的常用薄膜,因为它具有良好的灵敏度、整体稳定性和对 O2 的不敏感性。ASV 使用 Bi 薄膜以及镉 (Cd) 和铅 (Pb) 作为电镀介质,最近已用于测定强碱性环境(30% NaOH 或 35% M KOH)中的锌 (Zn) 浓度。碱性中锌的成功分析依赖于氢氧化物形成可溶性金属阴离子物质的能力,例如 Bi(OH)4- 和 Zn(OH)42-。在这里,我们希望扩展这项技术以检测和量化这些强碱性电解质中的铜 (Cu) 离子。然而,一般来说,使用 ASV 来检测和量化 Cu 离子浓度是出了名的困难,因为 Cu 剥离峰值电位与来自普通 Bi 膜电极的 Bi 电位重叠。在这里,利用铅作为沉积介质开发了一种用于测定碱性溶液中铜浓度的 ASV 方法。因此,发现在存在 Pb 的情况下分析 Cu 溶液时,溶出伏安曲线呈现单独且确定的 Cu 溶出峰。进行了不同的分析以找到最佳的溶出伏安法性能条件。因此,5 分钟的累积时间、≤-1.45 V vs. Hg/HgO 的累积电位和 35 wt% KOH 的浓度被确定为呈现最佳 ASV 结果的条件。利用这些条件,在 5. 0 ppm Pb 显示最佳线性溶出信号相关性,r 平方值为 0.991,检测限 (LOD) 为 0.67 ppm。这些结果让位于在碱性水溶液中使用 ASV 评估 Cu 浓度。
更新日期:2017-10-05
中文翻译:

通过铅介体使用阳极溶出伏安法检测碱性电解质中的铜
阳极溶出伏安法 (ASV) 是一种分析技术,可以对溶液中的金属离子种类进行选择性和定量分析。由于固有的金属离子溶解度,它最常用于中性至酸性电解质中。铋 (Bi) 是用于 ASV 的常用薄膜,因为它具有良好的灵敏度、整体稳定性和对 O2 的不敏感性。ASV 使用 Bi 薄膜以及镉 (Cd) 和铅 (Pb) 作为电镀介质,最近已用于测定强碱性环境(30% NaOH 或 35% M KOH)中的锌 (Zn) 浓度。碱性中锌的成功分析依赖于氢氧化物形成可溶性金属阴离子物质的能力,例如 Bi(OH)4- 和 Zn(OH)42-。在这里,我们希望扩展这项技术以检测和量化这些强碱性电解质中的铜 (Cu) 离子。然而,一般来说,使用 ASV 来检测和量化 Cu 离子浓度是出了名的困难,因为 Cu 剥离峰值电位与来自普通 Bi 膜电极的 Bi 电位重叠。在这里,利用铅作为沉积介质开发了一种用于测定碱性溶液中铜浓度的 ASV 方法。因此,发现在存在 Pb 的情况下分析 Cu 溶液时,溶出伏安曲线呈现单独且确定的 Cu 溶出峰。进行了不同的分析以找到最佳的溶出伏安法性能条件。因此,5 分钟的累积时间、≤-1.45 V vs. Hg/HgO 的累积电位和 35 wt% KOH 的浓度被确定为呈现最佳 ASV 结果的条件。利用这些条件,在 5. 0 ppm Pb 显示最佳线性溶出信号相关性,r 平方值为 0.991,检测限 (LOD) 为 0.67 ppm。这些结果让位于在碱性水溶液中使用 ASV 评估 Cu 浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号