当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Mechanical Roles for the C-Terminal Nonrepetitive Domain Become Apparent in Recombinant Spider Aciniform Silk
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-10-03 00:00:00 , DOI: 10.1021/acs.biomac.7b01057 Lingling Xu 1 , Thierry Lefèvre 2 , Kathleen E. Orrell , Qing Meng 1 , Michèle Auger 2 , Xiang-Qin Liu , Jan K. Rainey
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-10-03 00:00:00 , DOI: 10.1021/acs.biomac.7b01057 Lingling Xu 1 , Thierry Lefèvre 2 , Kathleen E. Orrell , Qing Meng 1 , Michèle Auger 2 , Xiang-Qin Liu , Jan K. Rainey
Affiliation
|
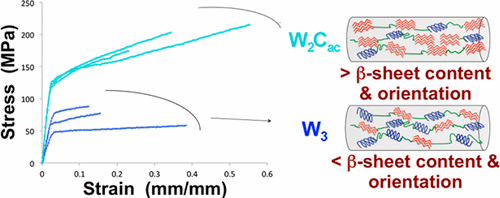
Spider aciniform (or wrapping) silk is the toughest of the seven types of spider silks/glue due to a combination of high elasticity and strength. Like most spider silk proteins (spidroins), aciniform spidroin (AcSp1) has a large core repetitive domain flanked by relatively short N- and C-terminal nonrepetitive domains (the NTD and CTD, respectively). The major ampullate silk protein (MaSp) CTD has been shown to control protein solubility and fiber formation, but the aciniform CTD function remains unknown. Here, we compare fiber mechanical properties, solution-state structuring, and fibrous state secondary structural composition, and orientation relative to native aciniform silk for two AcSp1 repeat units with or without fused AcSp1- and MaSp-derived CTDs alongside three AcSp1 repeat units without a CTD. The native AcSp1 CTD uniquely modulated fiber mechanical properties, relative to all other constructs, directly correlating to a native-like structural transformation and alignment.
中文翻译:

C末端非重复域的结构和机械作用在重组蜘蛛鹰嘴形丝中变得明显。
蜘蛛丝状(或包裹性)丝绸是七种类型的蜘蛛丝/胶中最坚韧的一种,因为它兼具高弹性和强度。像大多数蜘蛛丝蛋白(spidroins)一样,aciniform spidroin(AcSp1)具有较大的核心重复结构域,其侧翼是相对较短的N和C端非重复结构域(分别为NTD和CTD)。主要的壶腹丝蛋白(MaSp)CTD已显示可控制蛋白的溶解度和纤维形成,但腺状CTD的功能仍然未知。在这里,我们比较了两个AcSp1重复单元(带有或不带有融合的AcSp1-和MaSp衍生的CTD)以及三个AcSp1重复单元(不包含)的纤维力学性能,固溶态结构和纤维态二级结构组成,以及相对于天然腺状丝的取向CTD。
更新日期:2017-10-03
中文翻译:

C末端非重复域的结构和机械作用在重组蜘蛛鹰嘴形丝中变得明显。
蜘蛛丝状(或包裹性)丝绸是七种类型的蜘蛛丝/胶中最坚韧的一种,因为它兼具高弹性和强度。像大多数蜘蛛丝蛋白(spidroins)一样,aciniform spidroin(AcSp1)具有较大的核心重复结构域,其侧翼是相对较短的N和C端非重复结构域(分别为NTD和CTD)。主要的壶腹丝蛋白(MaSp)CTD已显示可控制蛋白的溶解度和纤维形成,但腺状CTD的功能仍然未知。在这里,我们比较了两个AcSp1重复单元(带有或不带有融合的AcSp1-和MaSp衍生的CTD)以及三个AcSp1重复单元(不包含)的纤维力学性能,固溶态结构和纤维态二级结构组成,以及相对于天然腺状丝的取向CTD。











































 京公网安备 11010802027423号
京公网安备 11010802027423号