当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Mechanism of Action of (−)-Lomaiviticin A
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1021/acs.accounts.7b00347 Seth B Herzon 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-09-28 00:00:00 , DOI: 10.1021/acs.accounts.7b00347 Seth B Herzon 1, 2
Affiliation
|
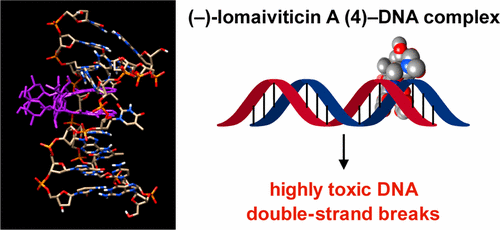
(−)-Lomaiviticin A (4) is a complex C2-symmetric bacterial metabolite that contains two diazofluorene functional groups. The diazofluorene consists of naphthoquinone, cyclopentadiene, and diazo substituents fused through a σ- and π-bonding network. Additionally, (−)-lomaiviticin A (4) is a potent cytotoxin, with half-maximal inhibitory potency (IC50) values in the low nanomolar range against many cancer cell lines. Because of limitations in supply, its mechanism of action had remained a “black box” since its isolation in the early 2000s. In this Account, I describe how studies directed toward the total synthesis of (−)-lomaiviticin A (4) provided a platform to elucidate the emergent properties of this metabolite and thereby connect chemical reactivity with cellular phenotype.
中文翻译:

(−)-Lomaiviticin A 的作用机制
(−)-Lomaiviticin A ( 4 ) 是一种复杂的C 2对称细菌代谢物,含有两个重氮芴官能团。重氮芴由萘醌、环戊二烯和通过σ-和π-键网络稠合的重氮取代基组成。此外,(−)-lomaiviticin A ( 4 ) 是一种有效的细胞毒素,对许多癌细胞系具有低纳摩尔范围内的半最大抑制效力 (IC 50 ) 值。由于供应有限,自2000年代初被隔离以来,其作用机制一直是“黑匣子”。在这篇文章中,我描述了针对 (−)-lomaiviticin A ( 4 ) 全合成的研究如何提供一个平台来阐明这种代谢物的新特性,从而将化学反应性与细胞表型联系起来。
更新日期:2017-09-28
中文翻译:

(−)-Lomaiviticin A 的作用机制
(−)-Lomaiviticin A ( 4 ) 是一种复杂的C 2对称细菌代谢物,含有两个重氮芴官能团。重氮芴由萘醌、环戊二烯和通过σ-和π-键网络稠合的重氮取代基组成。此外,(−)-lomaiviticin A ( 4 ) 是一种有效的细胞毒素,对许多癌细胞系具有低纳摩尔范围内的半最大抑制效力 (IC 50 ) 值。由于供应有限,自2000年代初被隔离以来,其作用机制一直是“黑匣子”。在这篇文章中,我描述了针对 (−)-lomaiviticin A ( 4 ) 全合成的研究如何提供一个平台来阐明这种代谢物的新特性,从而将化学反应性与细胞表型联系起来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号