当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron Transfer from Bi-Isonicotinic Acid Emerges upon Photodegradation of N3-Sensitized TiO2 Electrodes
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acsami.7b08986 Melike Karakus 1 , Wen Zhang 1 , Hans Joachim Räder 1 , Mischa Bonn 1 , Enrique Cánovas 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acsami.7b08986 Melike Karakus 1 , Wen Zhang 1 , Hans Joachim Räder 1 , Mischa Bonn 1 , Enrique Cánovas 1
Affiliation
|
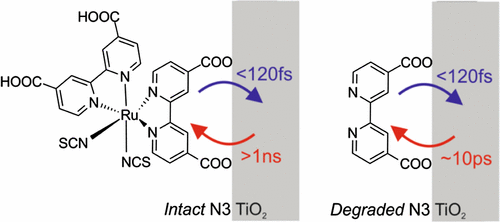
The long-term stability of dye-sensitized solar cells (DSSCs) is determined to a large extent by the photodegradation of their sensitizers. Understanding the mechanism of light-induced decomposition of dyes sensitizing a mesoporous oxide matrix may therefore contribute to solutions to increase the life span of DSSCs. Here, we investigate, using ultrafast terahertz photoconductivity measurements, the evolution of interfacial electron-transfer (ET) dynamics in Ru(4,4′-dicarboxylic acid-2,2′-bipyridine)2(NCS)2 (N3) dye-sensitized mesoporous TiO2 electrodes upon dye photodegradation. Under inert environment, interfacial ET dynamics do not change over time, indicating that the dye is stable and photodegradation is absent; the associated ET dynamics are characterized by a sub-100 fs rise of the photoconductivity, followed by long-lived (≫1 ns) electrons in the oxide electrode. When the N3–TiO2 sample is exposed to air under identical illumination conditions, dye photodegradation is evident from the disappearance of the optical absorption associated with the dye. Remarkably, approximately half of the sub-100 fs ET is observed to still occur but is followed by very rapid (∼10 ps) electron–hole recombination. Laser desorption/ionization mass spectrometry, attenuated total reflection–Fourier transform infrared, and terahertz photoconductivity analyses reveal that the photodegraded ET signal originates from the N3 dye photodegradation product as bi-isonicotinic acid (4,4′-dicarboxylic acid-2,2′-bipyridine), which remains bonded to the TiO2 surface via either bidentate chelation or bridging-type geometry.
中文翻译:

N3敏化的TiO 2电极光降解后,出现双异烟酸的电子转移。
染料敏化太阳能电池(DSSC)的长期稳定性在很大程度上取决于其敏化剂的光降解能力。因此,了解光致敏化介孔氧化物基质的染料分解的机理可能有助于增加DSSC寿命的解决方案。在这里,我们使用超快的太赫兹光电导率测量研究Ru(4,4'-di羧酸-2,2'-联吡啶)2(NCS)2(N3)染料中界面电子转移(ET)动力学的演变,敏化介孔TiO 2电极在染料光降解时。在惰性环境下,界面ET动力学不会随时间变化,表明该染料稳定且不存在光降解作用。相关的ET动力学的特征是光电导率上升到100 fs以下,随后在氧化物电极中出现长寿命(≫1 ns)的电子。当N3-TiO 2样品在相同的光照条件下暴露于空气中,从与染料相关的光吸收的消失中可以明显看出染料的光降解。值得注意的是,低于100 fs的ET大约有一半仍在发生,但随后非常快速(〜10 ps)的电子-空穴复合。激光解吸/电离质谱,衰减的全反射-傅立叶变换红外和太赫兹光电导分析表明,光降解的ET信号源自N3染料的光降解产物,即双异烟酸(4,4'-dicarboxy-2,2' -联吡啶),其通过二齿螯合或桥联型几何结构保持与TiO 2表面的键合。
更新日期:2017-09-26
中文翻译:

N3敏化的TiO 2电极光降解后,出现双异烟酸的电子转移。
染料敏化太阳能电池(DSSC)的长期稳定性在很大程度上取决于其敏化剂的光降解能力。因此,了解光致敏化介孔氧化物基质的染料分解的机理可能有助于增加DSSC寿命的解决方案。在这里,我们使用超快的太赫兹光电导率测量研究Ru(4,4'-di羧酸-2,2'-联吡啶)2(NCS)2(N3)染料中界面电子转移(ET)动力学的演变,敏化介孔TiO 2电极在染料光降解时。在惰性环境下,界面ET动力学不会随时间变化,表明该染料稳定且不存在光降解作用。相关的ET动力学的特征是光电导率上升到100 fs以下,随后在氧化物电极中出现长寿命(≫1 ns)的电子。当N3-TiO 2样品在相同的光照条件下暴露于空气中,从与染料相关的光吸收的消失中可以明显看出染料的光降解。值得注意的是,低于100 fs的ET大约有一半仍在发生,但随后非常快速(〜10 ps)的电子-空穴复合。激光解吸/电离质谱,衰减的全反射-傅立叶变换红外和太赫兹光电导分析表明,光降解的ET信号源自N3染料的光降解产物,即双异烟酸(4,4'-dicarboxy-2,2' -联吡啶),其通过二齿螯合或桥联型几何结构保持与TiO 2表面的键合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号