当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold-Decorated Porous Silicon Nanopillars for Targeted Hyperthermal Treatment of Bacterial Infections
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acsami.7b13278 Hashim Alhmoud 1 , Anna Cifuentes-Rius 1 , Bahman Delalat 1, 2 , David G. Lancaster 3 , Nicolas H. Voelcker 1, 2, 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acsami.7b13278 Hashim Alhmoud 1 , Anna Cifuentes-Rius 1 , Bahman Delalat 1, 2 , David G. Lancaster 3 , Nicolas H. Voelcker 1, 2, 4
Affiliation
|
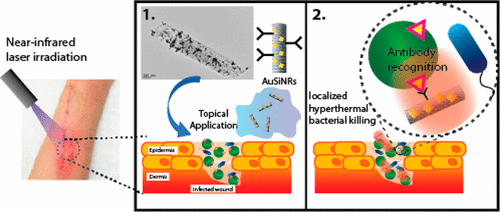
In order to address the issue of pathogenic bacterial colonization of diabetic wounds, a more direct and robust approach is required, which relies on a physical form of bacterial destruction in addition to the conventional biochemical approach (i.e., antibiotics). Targeted bacterial destruction through the use of photothermally active nanomaterials has recently come into the spotlight as a viable approach to solving the rising problem of antibiotic resistant microorganisms. Materials with high absorption coefficients in the near-infrared (NIR) region of the electromagnetic spectrum show promise as alternative antibacterial therapeutic agents, since they preclude the development of bacterial resistance and can be activated on demand. Here were report on a novel approach for the fabrication of gold nanoparticle decorated porous silicon nanopillars with tunable geometry that demonstrate excellent photothermal conversion properties when irradiated with a 808 nm laser. These photothermal antibacterial properties are demonstrated in vitro against the Gram-positive bacteria Staphylococcus aureus (S. aureus) and Gram-negative Escherichia coli (E. coli). Results show a reduction in bacterial viability of up to 99% after 10 min of laser irradiation. We also show an increase in antibacterial performance after modifying the nanopillars with S. aureus targeting antibodies causing up to a 10-fold increase in bactericidal efficiency compared to E. coli. In contrast, the nanomaterial resulted in minimal disruption of metabolic processes in human foreskin fibroblasts (HFF) after an equivalent period of irradiation.
中文翻译:

金装饰的多孔硅纳米柱用于细菌感染的靶向高温治疗。
为了解决糖尿病伤口的病原性细菌定植的问题,需要一种更直接和健壮的方法,除了常规的生化方法(即抗生素)之外,该方法还依赖于物理形式的细菌破坏。最近,通过使用光热活性纳米材料进行有针对性的细菌破坏已成为人们关注的解决细菌耐药性不断上升的问题的可行方法。在电磁波谱的近红外(NIR)区域具有高吸收系数的材料显示出有望用作替代抗菌治疗剂,因为它们可防止细菌耐药性的发展,并可按需激活。这是关于一种新颖的方法的报告,该方法用于制备具有可调几何形状的金纳米颗粒装饰的多孔硅纳米柱,当用808 nm激光辐照时,其表现出出色的光热转换性能。这些光热抗菌性能得到了证明在体外针对革兰氏阳性细菌金黄色葡萄球菌(S. aureus)和革兰氏阴性大肠杆菌(E. coli)。结果显示,在激光照射10分钟后,细菌活力降低了多达99%。我们还显示出用金黄色葡萄球菌靶向抗体修饰纳米柱后的抗菌性能增强,与大肠杆菌相比,其杀菌效率提高了10倍。相比之下,纳米材料在经过相同的照射时间后,对人类包皮成纤维细胞(HFF)的代谢过程造成的破坏最小。
更新日期:2017-09-26
中文翻译:

金装饰的多孔硅纳米柱用于细菌感染的靶向高温治疗。
为了解决糖尿病伤口的病原性细菌定植的问题,需要一种更直接和健壮的方法,除了常规的生化方法(即抗生素)之外,该方法还依赖于物理形式的细菌破坏。最近,通过使用光热活性纳米材料进行有针对性的细菌破坏已成为人们关注的解决细菌耐药性不断上升的问题的可行方法。在电磁波谱的近红外(NIR)区域具有高吸收系数的材料显示出有望用作替代抗菌治疗剂,因为它们可防止细菌耐药性的发展,并可按需激活。这是关于一种新颖的方法的报告,该方法用于制备具有可调几何形状的金纳米颗粒装饰的多孔硅纳米柱,当用808 nm激光辐照时,其表现出出色的光热转换性能。这些光热抗菌性能得到了证明在体外针对革兰氏阳性细菌金黄色葡萄球菌(S. aureus)和革兰氏阴性大肠杆菌(E. coli)。结果显示,在激光照射10分钟后,细菌活力降低了多达99%。我们还显示出用金黄色葡萄球菌靶向抗体修饰纳米柱后的抗菌性能增强,与大肠杆菌相比,其杀菌效率提高了10倍。相比之下,纳米材料在经过相同的照射时间后,对人类包皮成纤维细胞(HFF)的代谢过程造成的破坏最小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号