当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptide-Level Interactions between Proteins and Small-Molecule Drug Candidates by Two Hydrogen−Deuterium Exchange MS-Based Methods: The Example of Apolipoprotein E3
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.analchem.7b01121 Hanliu Wang 1, 2 , Don L. Rempel 1 , Daryl Giblin 1 , Carl Frieden 3 , Michael L. Gross 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.analchem.7b01121 Hanliu Wang 1, 2 , Don L. Rempel 1 , Daryl Giblin 1 , Carl Frieden 3 , Michael L. Gross 1
Affiliation
|
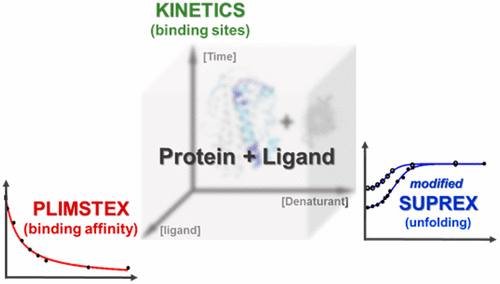
We describe a platform utilizing two methods based on hydrogen–deuterium exchange (HDX) coupled with mass spectrometry (MS) to characterize interactions between a protein and a small-molecule ligand. The model system is apolipoprotein E3 (apoE3) and a small-molecule drug candidate. We extended PLIMSTEX (protein–ligand interactions by mass spectrometry, titration, and H/D exchange) to the regional level by incorporating enzymatic digestion to acquire binding information for peptides. In a single experiment, we not only identified putative binding sites, but also obtained affinities of 6.0, 6.8, and 10.6 μM for the three different regions, giving an overall binding affinity of 7.4 μM. These values agree well with literature values determined by accepted methods. Unlike those methods, PLIMSTEX provides site-specific binding information. The second approach, modified SUPREX (stability of unpurified proteins from rates of H/D exchange) coupled with electrospray ionization (ESI), allowed us to obtain detailed understanding about apoE unfolding and its changes upon ligand binding. Three binding regions, along with an additional site, which may be important for lipid binding, show increased stability (less unfolding) upon ligand binding. By employing a single parameter, ΔC1/2%, we compared relative changes of denaturation between peptides. This integrated platform provides information orthogonal to commonly used HDX kinetics experiments, providing a general and novel approach for studying protein–ligand interactions.
中文翻译:

蛋白质和小分子候选药物之间基于两种氢氘交换质谱的方法之间的肽水平相互作用:载脂蛋白E3的实例
我们描述了一个平台,该平台利用基于氢-氘交换(HDX)和质谱(MS)的两种方法来表征蛋白质与小分子配体之间的相互作用。模型系统是载脂蛋白E3(apoE3)和小分子候选药物。我们通过结合酶消化来获取肽的结合信息,将PLIMSTEX(通过质谱,滴定和H / D交换进行的蛋白质-配体相互作用)扩展到了区域水平。在单个实验中,我们不仅确定了推定的结合位点,而且在三个不同区域获得了6.0、6.8和10.6μM的亲和力,总结合亲和力为7.4μM。这些值与通过公认方法确定的文献值非常吻合。与那些方法不同,PLIMSTEX提供了针对特定地点的绑定信息。第二种方法是修饰的SUPREX(来自H / D交换速率的未纯化蛋白的稳定性)与电喷雾电离(ESI)结合,使我们能够获得有关apoE展开及其在配体结合后的变化的详细了解。三个结合区域以及可能对脂质结合很重要的其他位点在配体结合后显示出增加的稳定性(较少的解折叠)。通过采用单个参数,Δ Ç 1/2%,我们比较了肽之间变性的相对变化。该集成平台提供了与常用的HDX动力学实验正交的信息,为研究蛋白质-配体相互作用提供了一种通用且新颖的方法。
更新日期:2017-09-26
中文翻译:

蛋白质和小分子候选药物之间基于两种氢氘交换质谱的方法之间的肽水平相互作用:载脂蛋白E3的实例
我们描述了一个平台,该平台利用基于氢-氘交换(HDX)和质谱(MS)的两种方法来表征蛋白质与小分子配体之间的相互作用。模型系统是载脂蛋白E3(apoE3)和小分子候选药物。我们通过结合酶消化来获取肽的结合信息,将PLIMSTEX(通过质谱,滴定和H / D交换进行的蛋白质-配体相互作用)扩展到了区域水平。在单个实验中,我们不仅确定了推定的结合位点,而且在三个不同区域获得了6.0、6.8和10.6μM的亲和力,总结合亲和力为7.4μM。这些值与通过公认方法确定的文献值非常吻合。与那些方法不同,PLIMSTEX提供了针对特定地点的绑定信息。第二种方法是修饰的SUPREX(来自H / D交换速率的未纯化蛋白的稳定性)与电喷雾电离(ESI)结合,使我们能够获得有关apoE展开及其在配体结合后的变化的详细了解。三个结合区域以及可能对脂质结合很重要的其他位点在配体结合后显示出增加的稳定性(较少的解折叠)。通过采用单个参数,Δ Ç 1/2%,我们比较了肽之间变性的相对变化。该集成平台提供了与常用的HDX动力学实验正交的信息,为研究蛋白质-配体相互作用提供了一种通用且新颖的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号