当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling Binding Interactions between Human RANKL and Its Decoy Receptor Osteoprotegerin
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.jpcb.7b06687 Aravinda Munasinghe 1 , Ping Lin 1 , Coray M. Colina 1, 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.jpcb.7b06687 Aravinda Munasinghe 1 , Ping Lin 1 , Coray M. Colina 1, 2
Affiliation
|
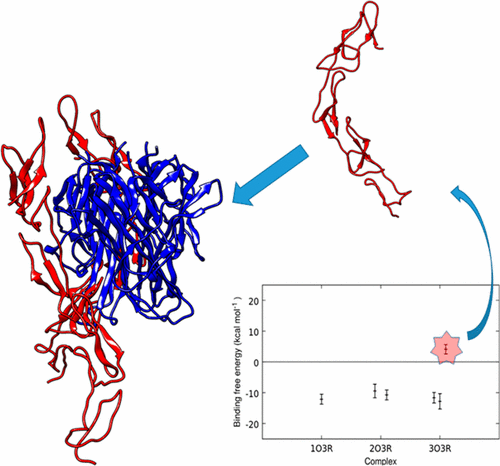
Recent studies have revealed the importance and the active contribution of the RANKL/OPG/RANK pathway in many bone diseases including different forms of common osteoporosis. In this study, we present an extensive atomistic molecular dynamic study of the OPG/RANKL system. Within the molecular models, we varied the number of OPG molecules bound to the RANKL trimer and carried out a study to determine how the binding affinity of the OPG/RANKL system changes as a function of OPG concentration. The molecular mechanics Poisson–Boltzmann surface area method was used to analyze binding free energies. It is shown that the binding affinity decreases with increasing numbers of OPG molecules. Additionally, conformational changes of RANKL, interactions between the N-terminus outlier module of OPG with RANKL, and residues that play an important role in the binding of OPG to RANKL trimer were investigated. A probable cause for unfavorable binding for a third OPG molecule was found. Along with the currently available experimental studies, this computational study will be valuable for the comprehensive understanding of OPG/RANKL at the atomistic level.
中文翻译:

揭示人类RANKL及其诱饵受体骨保护素之间的结合相互作用
最近的研究表明,RANKL / OPG / RANK途径在包括不同形式的常见骨质疏松症在内的许多骨骼疾病中的重要性和积极作用。在这项研究中,我们对OPG / RANKL系统进行了广泛的原子分子动力学研究。在分子模型中,我们改变了与RANKL三聚体结合的OPG分子的数量,并进行了一项研究以确定OPG / RANKL系统的结合亲和力如何随OPG浓度的变化而变化。分子力学泊松-玻尔兹曼表面积法用于分析结合自由能。结果表明,随着OPG分子数量的增加,结合亲和力降低。此外,RANKL的构象变化,OPG的N末端离群模块与RANKL之间的相互作用,研究了在OPG与RANKL三聚体结合中起重要作用的残基。发现了与第三OPG分子的不利结合的可能原因。连同当前可用的实验研究,该计算研究对于在原子级上对OPG / RANKL的全面理解将是有价值的。
更新日期:2017-09-25
中文翻译:

揭示人类RANKL及其诱饵受体骨保护素之间的结合相互作用
最近的研究表明,RANKL / OPG / RANK途径在包括不同形式的常见骨质疏松症在内的许多骨骼疾病中的重要性和积极作用。在这项研究中,我们对OPG / RANKL系统进行了广泛的原子分子动力学研究。在分子模型中,我们改变了与RANKL三聚体结合的OPG分子的数量,并进行了一项研究以确定OPG / RANKL系统的结合亲和力如何随OPG浓度的变化而变化。分子力学泊松-玻尔兹曼表面积法用于分析结合自由能。结果表明,随着OPG分子数量的增加,结合亲和力降低。此外,RANKL的构象变化,OPG的N末端离群模块与RANKL之间的相互作用,研究了在OPG与RANKL三聚体结合中起重要作用的残基。发现了与第三OPG分子的不利结合的可能原因。连同当前可用的实验研究,该计算研究对于在原子级上对OPG / RANKL的全面理解将是有价值的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号