当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalysis and Inhibition in the Electrochemical Reduction of CO2 on Platinum in the Presence of Protonated Pyridine. New Insights into Mechanisms and Products
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/jacs.7b08028 Hachem Dridi 1 , Clément Comminges 2 , Claudia Morais 2 , Jean-Claude Meledje 1 , Kouakou Boniface Kokoh 2 , Cyrille Costentin 1 , Jean-Michel Savéant 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/jacs.7b08028 Hachem Dridi 1 , Clément Comminges 2 , Claudia Morais 2 , Jean-Claude Meledje 1 , Kouakou Boniface Kokoh 2 , Cyrille Costentin 1 , Jean-Michel Savéant 1
Affiliation
|
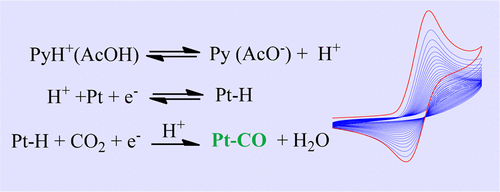
In the framework of modern energy challenges, the reduction of CO2 into fuels calls for electrogenerated low-valent transition metal complexes catalysts designed with considerable ingenuity and sophistication. For this reason, the report that a molecule as simple as protonated pyridine (PyH+) could catalyze the formation of methanol from the reduction of CO2 on a platinum electrode triggered great interest and excitement. Further investigations revealed that no methanol is produced. It appears that CO2 is not really reduced but rather participates, on the basis of its aquation into carbonic acid, in hydrogen evolution. Actually, the situation is not that straightforward, as revealed by scrutinizing what happens at the platinum electrode surface. The present study confirms the lack of methanol formation upon bulk electrolysis of PyH+ solutions at Pt and provides a detailed account of the Faradaic yield for H2 production as a function of the electrode potential, but the main finding is that CO2 reduction is accompanied by a strong inhibition of the electrode process taking place when it is carried out in the presence of acids such as PyH+ and AcOH. Cyclic voltammetry and in situ infrared spectroscopy were closely combined to investigate and understand the nature and consequences of the inhibition process. Constant comparison between the two acids was required to decipher the course of the reaction owing to the fact that the IR responses are perturbed by PyH+ adsorption. It finally appears that inhibition is caused by the reduction of CO2 into CO, whose high affinity with platinum triggers the formation of a Pt–CO film that prevents the reaction process. Thus, a paradoxical situation develops in which the high affinity of Pt for CO helps to decrease the overpotential for the reduction of CO2 and therefore blocks the electrode, preventing the reaction process.
中文翻译:

质子化吡啶存在下铂上CO 2电化学还原的催化和抑制作用 机制和产品的新见解
在现代能源挑战的框架内,将CO 2还原为燃料需要电生成的低价过渡金属络合物催化剂,该催化剂的设计必须具有独创性和复杂性。因此,关于质子化吡啶(PyH +)这样简单的分子可以催化铂电极上的CO 2还原而催化甲醇形成的报道引起了极大的兴趣和兴奋。进一步的调查表明,没有产生甲醇。似乎CO 2不会真正还原,而是根据其碳酸转化为氢的过程而参与还原。实际上,情况并非如此简单,正如仔细检查铂电极表面发生的情况所揭示的那样。本研究证实了在Pt大量电解PyH +溶液后甲醇形成的缺乏,并详细说明了H 2产生的法拉第产率与电极电位的关系,但主要发现是伴随着CO 2的还原通过在存在酸(例如PyH +和AcOH。循环伏安法和原位红外光谱法紧密结合,以研究和了解抑制过程的性质和后果。由于IR响应会受到PyH +吸附的干扰,因此需要对两种酸进行恒定的比较来解释反应的过程。最终看来,抑制作用是由CO 2还原为CO引起的,CO与铂的高亲和力触发了阻止反应过程的Pt-CO膜的形成。因此,出现了自相矛盾的情况,其中Pt对CO的高亲和力有助于减少用于CO 2还原的过电势,因此阻塞了电极,从而阻止了反应过程。
更新日期:2017-09-25
中文翻译:

质子化吡啶存在下铂上CO 2电化学还原的催化和抑制作用 机制和产品的新见解
在现代能源挑战的框架内,将CO 2还原为燃料需要电生成的低价过渡金属络合物催化剂,该催化剂的设计必须具有独创性和复杂性。因此,关于质子化吡啶(PyH +)这样简单的分子可以催化铂电极上的CO 2还原而催化甲醇形成的报道引起了极大的兴趣和兴奋。进一步的调查表明,没有产生甲醇。似乎CO 2不会真正还原,而是根据其碳酸转化为氢的过程而参与还原。实际上,情况并非如此简单,正如仔细检查铂电极表面发生的情况所揭示的那样。本研究证实了在Pt大量电解PyH +溶液后甲醇形成的缺乏,并详细说明了H 2产生的法拉第产率与电极电位的关系,但主要发现是伴随着CO 2的还原通过在存在酸(例如PyH +和AcOH。循环伏安法和原位红外光谱法紧密结合,以研究和了解抑制过程的性质和后果。由于IR响应会受到PyH +吸附的干扰,因此需要对两种酸进行恒定的比较来解释反应的过程。最终看来,抑制作用是由CO 2还原为CO引起的,CO与铂的高亲和力触发了阻止反应过程的Pt-CO膜的形成。因此,出现了自相矛盾的情况,其中Pt对CO的高亲和力有助于减少用于CO 2还原的过电势,因此阻塞了电极,从而阻止了反应过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号