当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibiting Mercury Re-emission and Enhancing Magnesia Recovery by Cobalt-Loaded Carbon Nanotubes in a Novel Magnesia Desulfurization Process
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.est.7b03364 Lidong Wang 1 , Tieyue Qi 1 , Mengxuan Hu 1 , Shihan Zhang 2 , Peiyao Xu 1 , Dan Qi 1 , Siyu Wu 1 , Huining Xiao 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.est.7b03364 Lidong Wang 1 , Tieyue Qi 1 , Mengxuan Hu 1 , Shihan Zhang 2 , Peiyao Xu 1 , Dan Qi 1 , Siyu Wu 1 , Huining Xiao 1
Affiliation
|
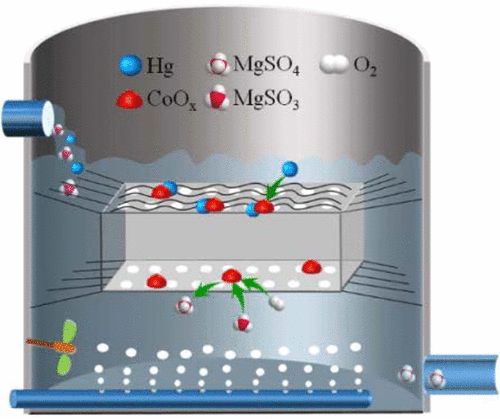
Mercury re-emission, because of the reduction of Hg2+ to form Hg0 by sulfite, has become a great concern in the desulfurization process. Lowering the concentrations of Hg2+ and sulfite in the desulfurization slurry can retard the Hg0 formation and, thus, mitigate mercury re-emission. To that end, cobalt-based carbon nanotubes (Co-CNTs) were developed for the simultaneous Hg2+ removal and sulfite oxidation in this work. Furthermore, the thermodynamics and kinetics of the Hg2+ adsorption and effect of Hg2+ adsorption on catalytic activity of Co-CNTs were investigated. Experimental results revealed that the Co-CNTs not only accelerated sulfite oxidation to enable the recovery of desulfurization by-products but also acted as an effective adsorbent of Hg2+ removal. The Hg2+ adsorption rate mainly depended on the structure of the adsorption material regardless of the cobalt loading and morphological distribution. The catalytic activity of the Co-CNTs for sulfite oxidation was not significantly affected due to the Hg2+ adsorption. Additionally, the isothermal adsorption behavior was well-fitted to the Langmuir model with an adsorption capacity of 166.7 mg/g. The mercury mass balance analysis revealed that the Hg0 re-emission was decreased by 156% by adding 2.0 g/L of Co-CNTs. These results can be used as a reference for the simultaneous removal of multiple pollutants in the wet-desulfurization process.
中文翻译:

通过新型的氧化镁脱硫工艺抑制汞的再排放并通过负载钴的碳纳米管增强氧化镁的回收率
水星再发射,因为减少汞的,2+形成汞0亚硫酸盐,已经成为脱硫工艺极大的关注。降低脱硫浆料中的Hg 2+和亚硫酸盐的浓度可以延迟Hg 0的形成,从而减轻汞的再排放。为此,开发了钴基碳纳米管(Co-CNT),用于在这项工作中同时去除Hg 2+和亚硫酸盐氧化。此外,热力学和汞的动力学2+的汞吸附和效果2+研究了吸附对Co-CNTs催化活性的影响。实验结果表明,Co-CNTs不仅可以加速亚硫酸盐的氧化,使脱硫副产物得以回收,而且还可以作为Hg 2+去除的有效吸附剂。Hg 2+的吸附速率主要取决于吸附材料的结构,而与钴的负载量和形态分布无关。由于Hg 2+的吸附,Co-CNTs对亚硫酸盐氧化的催化活性没有受到显着影响。此外,等温吸附行为非常适合Langmuir模型,吸附容量为166.7 mg / g。汞质量平衡分析表明,汞0通过添加2.0 g / L的Co-CNT,再排放减少了156%。这些结果可作为湿法脱硫过程中同时去除多种污染物的参考。
更新日期:2017-09-25
中文翻译:

通过新型的氧化镁脱硫工艺抑制汞的再排放并通过负载钴的碳纳米管增强氧化镁的回收率
水星再发射,因为减少汞的,2+形成汞0亚硫酸盐,已经成为脱硫工艺极大的关注。降低脱硫浆料中的Hg 2+和亚硫酸盐的浓度可以延迟Hg 0的形成,从而减轻汞的再排放。为此,开发了钴基碳纳米管(Co-CNT),用于在这项工作中同时去除Hg 2+和亚硫酸盐氧化。此外,热力学和汞的动力学2+的汞吸附和效果2+研究了吸附对Co-CNTs催化活性的影响。实验结果表明,Co-CNTs不仅可以加速亚硫酸盐的氧化,使脱硫副产物得以回收,而且还可以作为Hg 2+去除的有效吸附剂。Hg 2+的吸附速率主要取决于吸附材料的结构,而与钴的负载量和形态分布无关。由于Hg 2+的吸附,Co-CNTs对亚硫酸盐氧化的催化活性没有受到显着影响。此外,等温吸附行为非常适合Langmuir模型,吸附容量为166.7 mg / g。汞质量平衡分析表明,汞0通过添加2.0 g / L的Co-CNT,再排放减少了156%。这些结果可作为湿法脱硫过程中同时去除多种污染物的参考。










































 京公网安备 11010802027423号
京公网安备 11010802027423号