当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Significance of Surface Formate Coverage on the Reaction Kinetics of Methanol Synthesis from CO2 Hydrogenation over Cu
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acscatal.7b01910 Panpan Wu 1, 2, 3 , Bo Yang 1, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acscatal.7b01910 Panpan Wu 1, 2, 3 , Bo Yang 1, 4
Affiliation
|
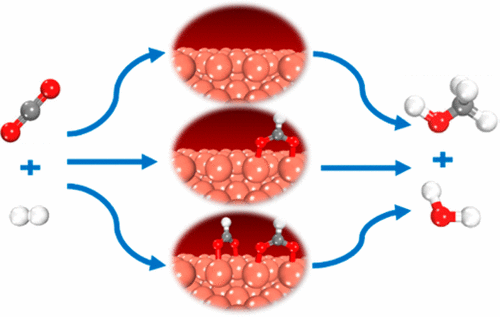
The hydrogenation of CO2 to methanol over copper-based catalysts has attracted considerable attention recently. Among all the proposed reaction mechanisms, a large number of experimental and theoretical studies have focused on the one that includes a HCOO intermediate due to the fact that high coverages of formate over catalyst surfaces were observed experimentally. To systematically understand the influence of formate species coverage on the reaction kinetics of methanol synthesis, the energetics of the CO2 hydrogenation pathway over clean and one- or two-formate preadsorbed Cu(211) are obtained using density functional theory calculations, and these energetics are further employed for microkinetic modeling. We find that the adsorption energies of the intermediates and transition states involved in the reaction pathway are changed in the presence of spectating formate species, and consequently, the potential energy diagrams are varied. Microkinetic analysis shows that the turnover frequencies (TOFs) over different formate preadsorbed surfaces vary under the same reaction condition. In particular, the reaction rates obtained over clean Cu(211) are generally the lowest, while those over one- or two-formate preadsorbed surfaces depend on the reaction temperatures and pressures. Meanwhile, we find that only when the formate coverage effect is considered, some of the TOFs obtained from microkinetic modeling are in fair agreement with previous experimental results under similar conditions. After the degree of rate control analysis, it is found that the combination of HCOO and HCOOH hydrogenation steps can be treated as the “effective rate-determining step”, which can be written as HCOO* + 2H* → H2COOH* + 2*. Therefore, the formation of methanol is mainly controlled by the surface coverage of formate and hydrogen at the steady state, as well as the free energy barriers of the effective rate-determining step, i.e., effective free energy barriers.
中文翻译:

铜的表面形态覆盖对CO 2加氢合成甲醇反应动力学的意义。
近来,在铜基催化剂上将CO 2加氢成甲醇引起了相当大的关注。在所有提出的反应机理中,由于实验观察到甲酸在催化剂表面的高覆盖率,因此大量的实验和理论研究都集中在包括HCOO中间体的反应机理上。为了系统地了解甲酸盐类化合物的覆盖对甲醇合成反应动力学(CO 2的能量)的影响使用密度泛函理论计算获得了在干净的和一或两个形式的预吸附铜(211)上的加氢途径,并将这些能量学进一步用于微动力学建模。我们发现,在存在甲酸的物质的存在下,反应路径中涉及的中间体和过渡态的吸附能发生了变化,因此势能图也发生了变化。微动力学分析表明,在相同的反应条件下,不同的甲酸盐预吸附表面上的周转频率(TOF)会发生变化。特别是,在干净的Cu(211)上获得的反应速率通常是最低的,而在一或二种形式的预吸附表面上获得的反应速率则取决于反应温度和压力。同时,我们发现只有考虑到甲酸覆盖效应,从微动力学模型获得的一些TOF与相似条件下的先前实验结果完全吻合。经过速率控制分析后,发现HCOO和HCOOH加氢步骤的组合可以看作是“有效的速率决定步骤”,可以写为HCOO * + 2H *→H2 COOH * + 2 *。因此,甲醇的形成主要受稳态下甲酸和氢的表面覆盖以及有效速率确定步骤的自由能垒,即有效自由能垒的控制。
更新日期:2017-09-25
中文翻译:

铜的表面形态覆盖对CO 2加氢合成甲醇反应动力学的意义。
近来,在铜基催化剂上将CO 2加氢成甲醇引起了相当大的关注。在所有提出的反应机理中,由于实验观察到甲酸在催化剂表面的高覆盖率,因此大量的实验和理论研究都集中在包括HCOO中间体的反应机理上。为了系统地了解甲酸盐类化合物的覆盖对甲醇合成反应动力学(CO 2的能量)的影响使用密度泛函理论计算获得了在干净的和一或两个形式的预吸附铜(211)上的加氢途径,并将这些能量学进一步用于微动力学建模。我们发现,在存在甲酸的物质的存在下,反应路径中涉及的中间体和过渡态的吸附能发生了变化,因此势能图也发生了变化。微动力学分析表明,在相同的反应条件下,不同的甲酸盐预吸附表面上的周转频率(TOF)会发生变化。特别是,在干净的Cu(211)上获得的反应速率通常是最低的,而在一或二种形式的预吸附表面上获得的反应速率则取决于反应温度和压力。同时,我们发现只有考虑到甲酸覆盖效应,从微动力学模型获得的一些TOF与相似条件下的先前实验结果完全吻合。经过速率控制分析后,发现HCOO和HCOOH加氢步骤的组合可以看作是“有效的速率决定步骤”,可以写为HCOO * + 2H *→H2 COOH * + 2 *。因此,甲醇的形成主要受稳态下甲酸和氢的表面覆盖以及有效速率确定步骤的自由能垒,即有效自由能垒的控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号