当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polymorphism of Lysozyme Condensates
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.jpcb.7b05425 Mohammad S. Safari 1 , Michael C. Byington 1 , Jacinta C. Conrad 1 , Peter G. Vekilov 1, 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-09-25 00:00:00 , DOI: 10.1021/acs.jpcb.7b05425 Mohammad S. Safari 1 , Michael C. Byington 1 , Jacinta C. Conrad 1 , Peter G. Vekilov 1, 2
Affiliation
|
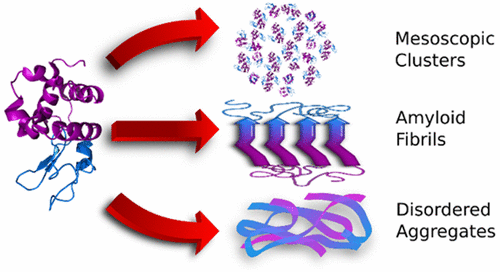
Protein condensates play essential roles in physiological processes and pathological conditions. Recently discovered mesoscopic protein-rich clusters may act as crucial precursors for the nucleation of ordered protein solids, such as crystals, sickle hemoglobin polymers, and amyloid fibrils. These clusters challenge settled paradigms of protein condensation as the constituent protein molecules present features characteristic of both partially misfolded and native proteins. Here we employ the antimicrobial enzyme lysozyme and examine the similarities between mesoscopic clusters, amyloid structures, and disordered aggregates consisting of chemically modified protein. We show that the mesoscopic clusters are distinct from the other two classes of aggregates. Whereas cluster formation and amyloid oligomerization are both reversible, aggregation triggered by reduction of the intramolecular S–S bonds is permanent. In contrast to the amyloid structures, protein molecules in the clusters retain their enzymatic activity. Furthermore, an essential feature of the mesoscopic clusters is their constant radius of less than 50 nm. The amyloid and disordered aggregates are significantly larger and rapidly grow. These findings demonstrate that the clusters are a product of limited protein structural flexibility. In view of the role of the clusters in the nucleation of ordered protein solids, our results suggest that fine-tuning the degree of protein conformational stability is a powerful tool to control and direct the pathways of protein condensation.
中文翻译:

溶菌酶缩合物的多态性
蛋白质冷凝物在生理过程和病理状况中起重要作用。最近发现的介观的富含蛋白质的簇可以充当有序蛋白质固体(例如晶体,镰状血红蛋白聚合物和淀粉样蛋白原纤维)成核的关键前体。这些簇挑战蛋白质缩合的稳定范式,因为组成蛋白质分子呈现出部分错误折叠的蛋白质和天然蛋白质的特征。在这里,我们使用抗微生物酶的溶菌酶,并检查介观簇,淀粉样蛋白结构和由化学修饰的蛋白质组成的无序聚集体之间的相似性。我们表明介观群集是不同于其他两个类别的聚合。簇的形成和淀粉样蛋白的低聚都是可逆的,分子内S–S键减少引起的聚集是永久性的。与淀粉样蛋白结构相反,簇中的蛋白质分子保留了其酶促活性。此外,介观簇的基本特征是它们的恒定半径小于50nm。淀粉样蛋白和无序的聚集体明显更大并且迅速生长。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。簇中的蛋白质分子保留其酶活性。此外,介观簇的基本特征是它们的恒定半径小于50nm。淀粉样蛋白和无序的聚集体明显更大并且迅速生长。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。簇中的蛋白质分子保留其酶活性。此外,介观簇的基本特征是它们的恒定半径小于50nm。淀粉样蛋白和无序的聚集体明显更大并且迅速生长。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。
更新日期:2017-09-25
中文翻译:

溶菌酶缩合物的多态性
蛋白质冷凝物在生理过程和病理状况中起重要作用。最近发现的介观的富含蛋白质的簇可以充当有序蛋白质固体(例如晶体,镰状血红蛋白聚合物和淀粉样蛋白原纤维)成核的关键前体。这些簇挑战蛋白质缩合的稳定范式,因为组成蛋白质分子呈现出部分错误折叠的蛋白质和天然蛋白质的特征。在这里,我们使用抗微生物酶的溶菌酶,并检查介观簇,淀粉样蛋白结构和由化学修饰的蛋白质组成的无序聚集体之间的相似性。我们表明介观群集是不同于其他两个类别的聚合。簇的形成和淀粉样蛋白的低聚都是可逆的,分子内S–S键减少引起的聚集是永久性的。与淀粉样蛋白结构相反,簇中的蛋白质分子保留了其酶促活性。此外,介观簇的基本特征是它们的恒定半径小于50nm。淀粉样蛋白和无序的聚集体明显更大并且迅速生长。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。簇中的蛋白质分子保留其酶活性。此外,介观簇的基本特征是它们的恒定半径小于50nm。淀粉样蛋白和无序的聚集体明显更大并且迅速生长。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。簇中的蛋白质分子保留其酶活性。此外,介观簇的基本特征是它们的恒定半径小于50nm。淀粉样蛋白和无序的聚集体明显更大并且迅速生长。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。这些发现表明,簇是有限的蛋白质结构灵活性的产物。考虑到簇在有序蛋白质固体成核中的作用,我们的结果表明,微调蛋白质构象稳定性的程度是控制和指导蛋白质浓缩途径的有力工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号