Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of drug combination for glioblastoma based on an intestine–liver metabolic model on microchip
Analyst ( IF 3.6 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7an00453b Mingsha Jie 1, 2, 3, 4, 5 , Sifeng Mao 5, 6, 7, 8, 9 , Hanyang Liu 5, 6, 7, 8, 9 , Ziyi He 5, 6, 7, 8, 9 , Hai-Fang Li 5, 6, 7, 8, 9 , Jin-Ming Lin 5, 6, 7, 8, 9
Analyst ( IF 3.6 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7an00453b Mingsha Jie 1, 2, 3, 4, 5 , Sifeng Mao 5, 6, 7, 8, 9 , Hanyang Liu 5, 6, 7, 8, 9 , Ziyi He 5, 6, 7, 8, 9 , Hai-Fang Li 5, 6, 7, 8, 9 , Jin-Ming Lin 5, 6, 7, 8, 9
Affiliation
|
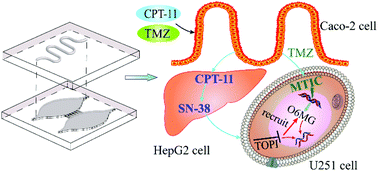
An intestine–liver–glioblastoma biomimetic system was developed to evaluate the drug combination therapy for glioblastoma. A hollow fiber (HF) was embedded into the upper layer of the microfluidic chip for culturing Caco-2 cells to mimic drug delivery as an artificial intestine. HepG2 cells cultured in the bottom chamber of the chip acted as an artificial liver for metabolizing the drugs. The dual-drug combination to glioblastoma U251 cells was evaluated based on the intestine–liver metabolic model. The drugs, irinotecan (CPT-11), temozolomide (TMZ) and cyclophosphamide (CP), were used to dynamically stimulate the cells by continuous infusion into the intestine unit. After intestine absorption and liver metabolism, the prodrugs were transformed to active metabolites, which induced glioblastoma cells apoptosis. The anticancer activity of the CPT-11 and TMZ combination is significantly enhanced compared to that of the single drug treatments. Combination index (CI) values of the combination groups, CPT-11 and TMZ, CPT-11 and CP, and TMZ and CP, at half maximal inhibitory concentration were 0.137, 0.288, and 0.482, respectively. The results indicated that the CPT-11 and TMZ combination was superior to the CPT-11 and CP group as well as the TMZ and CP group towards the U251 cells. The metabolism mechanism of CPT-11 and TMZ was further studied by coupling with mass spectrometric analysis. The biomimetic model enables the performance of long-term cell co-culture, drug delivery, metabolism and real-time analysis of drug effects, promising systematic in vitro mimicking of physiological and pharmacological processes.
中文翻译:

基于微芯片上肠道-肝脏代谢模型的胶质母细胞瘤药物组合评估
开发了肠-肝-胶质母细胞瘤仿生系统,以评估胶质母细胞瘤的药物联合治疗。中空纤维(HF)嵌入微流控芯片的上层,用于培养Caco-2细胞以模拟作为人工肠的药物输送。在芯片底部腔室中培养的HepG2细胞充当了用于代谢药物的人造肝脏。根据肠-肝代谢模型评估了对胶质母细胞瘤U251细胞的双重药物联合治疗。伊立替康(CPT-11),替莫唑胺(TMZ)和环磷酰胺(CP)等药物用于通过连续输注至肠道单位来动态刺激细胞。在肠道吸收和肝脏代谢后,前药转化为活性代谢物,从而诱导胶质母细胞瘤细胞凋亡。与单一药物治疗相比,CPT-11和TMZ组合的抗癌活性显着增强。CPT-11和TMZ,CPT-11和CP以及TMZ和CP在最大抑制浓度一半时的组合指数(CI)值分别为0.137、0.288和0.482。结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 CPT-11和TMZ,CPT-11和CP以及TMZ和CP在最大抑制浓度一半时的组合指数(CI)值分别为0.137、0.288和0.482。结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 CPT-11和TMZ,CPT-11和CP以及TMZ和CP在最大抑制浓度一半时的组合指数(CI)值分别为0.137、0.288和0.482。结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化在体外模仿生理和药理过程。
更新日期:2017-09-25
中文翻译:

基于微芯片上肠道-肝脏代谢模型的胶质母细胞瘤药物组合评估
开发了肠-肝-胶质母细胞瘤仿生系统,以评估胶质母细胞瘤的药物联合治疗。中空纤维(HF)嵌入微流控芯片的上层,用于培养Caco-2细胞以模拟作为人工肠的药物输送。在芯片底部腔室中培养的HepG2细胞充当了用于代谢药物的人造肝脏。根据肠-肝代谢模型评估了对胶质母细胞瘤U251细胞的双重药物联合治疗。伊立替康(CPT-11),替莫唑胺(TMZ)和环磷酰胺(CP)等药物用于通过连续输注至肠道单位来动态刺激细胞。在肠道吸收和肝脏代谢后,前药转化为活性代谢物,从而诱导胶质母细胞瘤细胞凋亡。与单一药物治疗相比,CPT-11和TMZ组合的抗癌活性显着增强。CPT-11和TMZ,CPT-11和CP以及TMZ和CP在最大抑制浓度一半时的组合指数(CI)值分别为0.137、0.288和0.482。结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 CPT-11和TMZ,CPT-11和CP以及TMZ和CP在最大抑制浓度一半时的组合指数(CI)值分别为0.137、0.288和0.482。结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 CPT-11和TMZ,CPT-11和CP以及TMZ和CP在最大抑制浓度一半时的组合指数(CI)值分别为0.137、0.288和0.482。结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化 结果表明,对于U251细胞,CPT-11和TMZ组合优于CPT-11和CP组以及TMZ和CP组。通过与质谱分析相结合,进一步研究了CPT-11和TMZ的代谢机理。仿生模型可实现长期细胞共培养,药物递送,新陈代谢和药物作用的实时分析,有望实现系统化在体外模仿生理和药理过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号