当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nonexponential kinetics of ion pair dissociation in electrofreezing water
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1039/c7cp04572g Mohammad Alaghemandi 1, 2, 3, 4 , Volkmar Koller 1, 1, 2, 3, 4 , Jason R. Green 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1039/c7cp04572g Mohammad Alaghemandi 1, 2, 3, 4 , Volkmar Koller 1, 1, 2, 3, 4 , Jason R. Green 1, 2, 3, 4, 5
Affiliation
|
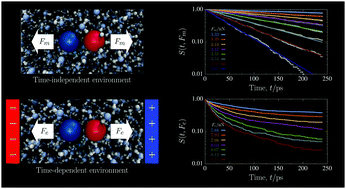
Temporally- or spatially-heterogeneous environments can participate in many kinetic processes, from chemical reactions and self-assembly to the forced dissociation of biomolecules. Here, we simulate the molecular dynamics of a model ion pair forced to dissociate in an explicit, aqueous solution. Triggering dissociation with an external electric field causes the surrounding water to electrofreeze and the ion pair population to decay nonexponentially. To further probe the role of the aqueous environment in the kinetics, we also simulate dissociation events under a purely mechanical force on the ion pair. In this case, regardless of whether the surrounding water is a liquid or already electrofrozen, the ion pair population decays exponentially with a well-defined rate constant that is specific to the medium and applied force. These simulation data, and the rate parameters we extract, suggest the disordered kinetics in an electrofreezing medium are a result of the comparable time scales of two concurrent processes, electrofreezing and dissociation.
中文翻译:

电冻结水中离子对解离的非指数动力学
时间或空间异质环境可以参与许多动力学过程,从化学反应和自组装到生物分子的强制解离。在这里,我们模拟了在明确的水溶液中被迫解离的模型离子对的分子动力学。触发与外部电场的离解会导致周围的水发生电冻结,并且离子对的数量会以非指数方式衰减。为了进一步探讨水环境在动力学中的作用,我们还模拟了在离子对的纯机械力作用下的解离事件。在这种情况下,无论周围的水是液态的还是已经电冻结的,离子对数量都以特定于介质和作用力的明确定义的速率常数呈指数衰减。这些模拟数据
更新日期:2017-09-25
中文翻译:

电冻结水中离子对解离的非指数动力学
时间或空间异质环境可以参与许多动力学过程,从化学反应和自组装到生物分子的强制解离。在这里,我们模拟了在明确的水溶液中被迫解离的模型离子对的分子动力学。触发与外部电场的离解会导致周围的水发生电冻结,并且离子对的数量会以非指数方式衰减。为了进一步探讨水环境在动力学中的作用,我们还模拟了在离子对的纯机械力作用下的解离事件。在这种情况下,无论周围的水是液态的还是已经电冻结的,离子对数量都以特定于介质和作用力的明确定义的速率常数呈指数衰减。这些模拟数据











































 京公网安备 11010802027423号
京公网安备 11010802027423号