当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A topological study of chemical bonds under pressure: solid hydrogen as a model case
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7cp04349j Vanessa Riffet 1, 2, 3, 4, 5 , Vanessa Labet 1, 2, 3, 6, 7 , Julia Contreras-García 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7cp04349j Vanessa Riffet 1, 2, 3, 4, 5 , Vanessa Labet 1, 2, 3, 6, 7 , Julia Contreras-García 1, 2, 3, 4, 5
Affiliation
|
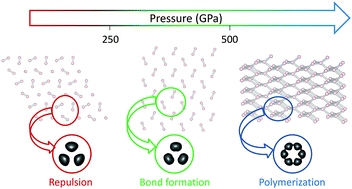
It is now well recognized that a fundamental understanding of the rules that govern chemistry under pressure is still lacking. Hydrogen being the “simplest” element as well as a central core to high pressure physics, we undertake a general study of the changes in the chemical bonding under pressure. We start from a simple trimer unit that has been found in high pressure phases, whose behavior has been found to reveal the basics of hydrogen polymerization under pressure. Making use of bond analysis tools, mainly the NCI (noncovalent interactions) index, we show that polymerization takes place in three steps: dipolar attraction, repulsion and bond formation. The use of a 1D Wigner–Seitz radius allowed us to extend the conclusions to 3D networks and to analyze their degree of polymerization. On the one hand, this approach provides new insight into the polymerization of hydrogen. On the other hand, it shows that complicated molecular solids can be understood from cluster models, where correlated methods can be applied, main differences in solid state arising at the transition points, where breaking/forming of bonds happens at once instead of continuously like in the cluster model.
中文翻译:

压力下化学键的拓扑研究:以固体氢为模型案例
现在已经众所周知,仍然缺乏对在压力下控制化学的规则的基本理解。氢是“最简单”的元素,也是高压物理学的核心,我们对压力下化学键的变化进行了全面的研究。我们从在高压相中发现的简单三聚体单元开始,发现其行为揭示了在压力下氢气聚合的基本原理。利用主要是NCI(非共价相互作用)指数的键分析工具,我们表明聚合反应分为三个步骤:偶极吸引,排斥和键形成。使用1D Wigner-Seitz半径可使我们将结论扩展到3D网络并分析其聚合度。一方面,这种方法为氢的聚合提供了新的见识。另一方面,它表明可以从聚类模型中理解复杂的分子固体,在聚类模型中可以应用相关方法,固态主要差异出现在过渡点,其中键的断裂/形成立即发生,而不是像集群模型。
更新日期:2017-09-25
中文翻译:

压力下化学键的拓扑研究:以固体氢为模型案例
现在已经众所周知,仍然缺乏对在压力下控制化学的规则的基本理解。氢是“最简单”的元素,也是高压物理学的核心,我们对压力下化学键的变化进行了全面的研究。我们从在高压相中发现的简单三聚体单元开始,发现其行为揭示了在压力下氢气聚合的基本原理。利用主要是NCI(非共价相互作用)指数的键分析工具,我们表明聚合反应分为三个步骤:偶极吸引,排斥和键形成。使用1D Wigner-Seitz半径可使我们将结论扩展到3D网络并分析其聚合度。一方面,这种方法为氢的聚合提供了新的见识。另一方面,它表明可以从聚类模型中理解复杂的分子固体,在聚类模型中可以应用相关方法,固态主要差异出现在过渡点,其中键的断裂/形成立即发生,而不是像集群模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号