当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combining the catalytic enantioselective reaction of visible-light-generated radicals with a by-product utilization system
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1039/c7sc02621h Xiaoqiang Huang 1, 2, 3, 4 , Shipeng Luo 1, 2, 3, 4 , Olaf Burghaus 1, 2, 3, 4 , Richard D. Webster 5, 6, 7, 8, 9 , Klaus Harms 1, 2, 3, 4 , Eric Meggers 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1039/c7sc02621h Xiaoqiang Huang 1, 2, 3, 4 , Shipeng Luo 1, 2, 3, 4 , Olaf Burghaus 1, 2, 3, 4 , Richard D. Webster 5, 6, 7, 8, 9 , Klaus Harms 1, 2, 3, 4 , Eric Meggers 1, 2, 3, 4
Affiliation
|
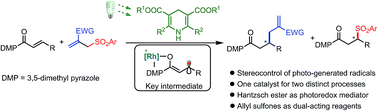
We report an unusual reaction design in which a chiral bis-cyclometalated rhodium(III) complex enables the stereocontrolled chemistry of photo-generated carbon-centered radicals and at the same time catalyzes an enantioselective sulfonyl radical addition to an alkene. Specifically, employing inexpensive and readily available Hantzsch esters as the photoredox mediator, Rh-coordinated prochiral radicals generated by a selective photoinduced single electron reduction are trapped by allyl sulfones in a highly stereocontrolled fashion, providing radical allylation products with up to 97% ee. The hereby formed fragmented sulfonyl radicals are utilized via an enantioselective radical addition to form chiral sulfones, which minimizes waste generation.
中文翻译:

结合可见光产生的自由基的催化对映选择性反应和副产物利用系统
我们报告了一种不寻常的反应设计,其中手性双环金属化铑(III)配合物使光生碳中心自由基的立体控制化学反应同时催化对烯的对映选择性磺酰基自由基加成。具体而言,使用廉价且容易获得的汉茨酯作为光氧化还原介体,由选择性光诱导的单电子还原生成的Rh配位的手性自由基被烯丙基砜以高度立体可控的方式捕获,从而提供了具有高达97%ee的自由基烯丙基化产物。由此形成的片段化的磺酰基自由基通过对映选择性自由基加成被利用以形成手性砜,这使废物的产生最小化。
更新日期:2017-09-25
中文翻译:

结合可见光产生的自由基的催化对映选择性反应和副产物利用系统
我们报告了一种不寻常的反应设计,其中手性双环金属化铑(III)配合物使光生碳中心自由基的立体控制化学反应同时催化对烯的对映选择性磺酰基自由基加成。具体而言,使用廉价且容易获得的汉茨酯作为光氧化还原介体,由选择性光诱导的单电子还原生成的Rh配位的手性自由基被烯丙基砜以高度立体可控的方式捕获,从而提供了具有高达97%ee的自由基烯丙基化产物。由此形成的片段化的磺酰基自由基通过对映选择性自由基加成被利用以形成手性砜,这使废物的产生最小化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号