当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total syntheses of schilancidilactones A and B, schilancitrilactone A, and 20-epi-schilancitrilactone A via late-stage nickel-catalyzed cross coupling
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7sc03293e Hengtao Wang 1, 2, 3, 4, 5 , Xiunan Zhang 1, 2, 3, 4, 5 , Pingping Tang 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1039/c7sc03293e Hengtao Wang 1, 2, 3, 4, 5 , Xiunan Zhang 1, 2, 3, 4, 5 , Pingping Tang 1, 2, 3, 4, 5
Affiliation
|
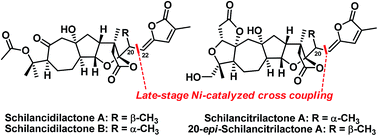
The first total syntheses of schilancidilactones A and B, schilancitrilactone A, and 20-epi-schilancitrilactone A have been accomplished using a nickel-catalyzed cross coupling of alkyl bromide with vinyl stannane as the final step. The other key steps include late-stage C(sp3)–H bromination, the oxidative cleavage of a diol to provide the requisite ketone and ester for schilancidilactones A and B, and Dieckmann-type condensation to generate the A ring of schilancitrilactone A and 20-epi-schilancitrilactone A.
中文翻译:

schilancidilactones A和B,schilancitrilactone A的全合成,和20-外延-schilancitrilactone甲经由后期镍-催化的交叉偶联
使用镍催化的烷基溴化物与乙烯基锡烷的交叉偶联,完成了Schilancidilactones A和B,Schilancitrilaclactone A和20- epi- schilancitrilactonetone A的第一批总合成。其他关键步骤包括后期C(sp 3)–H溴化,二醇的氧化裂解以提供Schilancidilactones A和B所需的酮和酯,以及Dieckmann型缩合反应生成Schilancitrilaclactone A和A的A环。 20- Epi- schilancitrilactonetone A.
更新日期:2017-09-25
中文翻译:

schilancidilactones A和B,schilancitrilactone A的全合成,和20-外延-schilancitrilactone甲经由后期镍-催化的交叉偶联
使用镍催化的烷基溴化物与乙烯基锡烷的交叉偶联,完成了Schilancidilactones A和B,Schilancitrilaclactone A和20- epi- schilancitrilactonetone A的第一批总合成。其他关键步骤包括后期C(sp 3)–H溴化,二醇的氧化裂解以提供Schilancidilactones A和B所需的酮和酯,以及Dieckmann型缩合反应生成Schilancitrilaclactone A和A的A环。 20- Epi- schilancitrilactonetone A.











































 京公网安备 11010802027423号
京公网安备 11010802027423号