当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Nazarov cyclization of indole enones cooperatively catalyzed by Lewis acids and chiral Brønsted acids
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1039/c7sc03183a Guo-Peng Wang 1, 2, 3, 4 , Meng-Qing Chen 1, 2, 3, 4 , Shou-Fei Zhu 1, 2, 3, 4 , Qi-Lin Zhou 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1039/c7sc03183a Guo-Peng Wang 1, 2, 3, 4 , Meng-Qing Chen 1, 2, 3, 4 , Shou-Fei Zhu 1, 2, 3, 4 , Qi-Lin Zhou 1, 2, 3, 4, 5
Affiliation
|
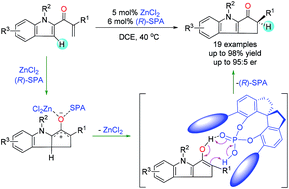
Enantioselective control of the chirality of a tertiary α-carbon in the products of a Nazarov cyclization of enones is challenging because the reaction involves an enantioselective proton transfer process. We herein report the use of cooperative catalysis using Lewis acids and chiral Brønsted acids to control the stereochemistry of the tertiary α-carbon in the products of this reaction. Specifically, with ZnCl2 and a chiral spiro phosphoric acid as catalysts, we realized the first enantioselective construction of cyclopenta[b]indoles with chiral tertiary α-carbons via Nazarov cyclization of indole enone substrates with only one coordinating site. Mechanistic studies revealed that the chiral spiro phosphoric acid acts as a multifunctional catalyst: it co-catalyzes the cyclization of the dienone and enantioselectively catalyzes a proton transfer reaction of the enol intermediate. This new strategy of enantioselective control by means of cooperative catalysis may show utility for other challenging asymmetric cyclization reactions.
中文翻译:

路易斯酸和手性布朗斯台德酸协同催化的吲哚烯酮的对映选择性纳扎罗夫环化反应
在纳扎罗夫环烯酮的产物中叔α-碳的手性的对映选择性控制是具有挑战性的,因为该反应涉及对映选择性质子转移过程。我们在本文中报道了使用路易斯酸和手性布朗斯台德酸的协同催化来控制该反应产物中叔α-碳的立体化学。具体而言,与氯化锌2和手性螺环磷酸作为催化剂,我们意识到环戊二烯并[第一对映选择性施工b〕吲哚与手性叔α-碳通过仅有一个配位位点的吲哚烯酮底物的纳扎罗夫环化。机理研究表明,手性螺磷酸可作为多功能催化剂:共催化二烯酮的环化反应,对映选择性催化烯醇中间体的质子转移反应。这种通过协同催化进行对映选择性控制的新策略可能显示出对其他具有挑战性的不对称环化反应的实用性。
更新日期:2017-09-25
中文翻译:

路易斯酸和手性布朗斯台德酸协同催化的吲哚烯酮的对映选择性纳扎罗夫环化反应
在纳扎罗夫环烯酮的产物中叔α-碳的手性的对映选择性控制是具有挑战性的,因为该反应涉及对映选择性质子转移过程。我们在本文中报道了使用路易斯酸和手性布朗斯台德酸的协同催化来控制该反应产物中叔α-碳的立体化学。具体而言,与氯化锌2和手性螺环磷酸作为催化剂,我们意识到环戊二烯并[第一对映选择性施工b〕吲哚与手性叔α-碳通过仅有一个配位位点的吲哚烯酮底物的纳扎罗夫环化。机理研究表明,手性螺磷酸可作为多功能催化剂:共催化二烯酮的环化反应,对映选择性催化烯醇中间体的质子转移反应。这种通过协同催化进行对映选择性控制的新策略可能显示出对其他具有挑战性的不对称环化反应的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号