当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic analysis of a copper-catalyzed C–H oxidative cyclization of carboxylic acids
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7sc02240a Shibdas Banerjee 1, 2, 3, 4 , Shyam Sathyamoorthi 1, 2, 3, 4 , J. Du Bois 1, 2, 3, 4 , Richard N. Zare 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-16 00:00:00 , DOI: 10.1039/c7sc02240a Shibdas Banerjee 1, 2, 3, 4 , Shyam Sathyamoorthi 1, 2, 3, 4 , J. Du Bois 1, 2, 3, 4 , Richard N. Zare 1, 2, 3, 4
Affiliation
|
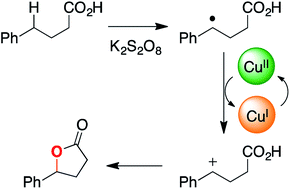
We recently reported that carboxylic acids can be oxidized to lactone products by potassium persulfate and catalytic copper acetate. Here, we unravel the mechanism for this C–H functionalization reaction using desorption electrospray ionization, online electrospray ionization, and tandem mass spectrometry. Our findings suggest that electron transfer from a transient benzylic radical intermediate reduces Cu(II) to Cu(I), which is then re-oxidized to Cu(II) in the catalytic cycle. The resulting benzylic carbocation is trapped by the pendant carboxylate group to give the lactone product. Formation of the putative benzylic carbocation is supported by Hammett analysis. The proposed mechanism for this copper-catalyzed oxidative cyclization process differs from earlier reports of analogous reactions, which posit a substrate carboxylate radical as the reactive oxidant.
中文翻译:

铜催化羧酸的C–H氧化环化的机理分析
我们最近报道,过硫酸钾和催化乙酸铜可将羧酸氧化为内酯产物。在这里,我们使用解吸电喷雾电离,在线电喷雾电离和串联质谱分析了该C–H功能化反应的机理。我们的发现表明,电子从瞬态苄基中间体的转移将Cu(II)还原为Cu(I),然后再被氧化为Cu(II))在催化循环中。所得的苄基碳正阳离子被侧链羧酸酯基团捕获,得到内酯产物。Hammett分析支持推定的苄基碳正离子的形成。铜催化的氧化环化过程的拟议机理与早期报道的类似反应不同,后者将底物羧酸根作为反应性氧化剂。
更新日期:2017-09-25
中文翻译:

铜催化羧酸的C–H氧化环化的机理分析
我们最近报道,过硫酸钾和催化乙酸铜可将羧酸氧化为内酯产物。在这里,我们使用解吸电喷雾电离,在线电喷雾电离和串联质谱分析了该C–H功能化反应的机理。我们的发现表明,电子从瞬态苄基中间体的转移将Cu(II)还原为Cu(I),然后再被氧化为Cu(II))在催化循环中。所得的苄基碳正阳离子被侧链羧酸酯基团捕获,得到内酯产物。Hammett分析支持推定的苄基碳正离子的形成。铜催化的氧化环化过程的拟议机理与早期报道的类似反应不同,后者将底物羧酸根作为反应性氧化剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号