当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-catalyzed cyanothiolation to incorporate a sulfur-substituted quaternary carbon center
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7sc02867a Yubing Huang 1, 2, 3, 4, 5 , Xianwei Li 1, 2, 3, 4, 5 , Xu Wang 1, 2, 3, 4, 5 , Yue Yu 1, 2, 3, 4, 5 , Jia Zheng 1, 2, 3, 4, 5 , Wanqing Wu 1, 2, 3, 4, 5 , Huanfeng Jiang 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7sc02867a Yubing Huang 1, 2, 3, 4, 5 , Xianwei Li 1, 2, 3, 4, 5 , Xu Wang 1, 2, 3, 4, 5 , Yue Yu 1, 2, 3, 4, 5 , Jia Zheng 1, 2, 3, 4, 5 , Wanqing Wu 1, 2, 3, 4, 5 , Huanfeng Jiang 1, 2, 3, 4, 5
Affiliation
|
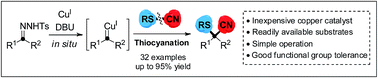
Sulfur-containing nitriles have important research value in the life sciences due to their diverse biological activities resulting from the sulfur and cyano functional groups. Herein, a copper-catalyzed cyanothiolation of N-tosylhydrazones with thiocyanates to generate α-arylthioalkanenitriles bearing sulfur-substituted quaternary carbon center atoms has been described. This novel protocol involves the procedure of copper carbene species promoting S–CN bond cleavage and C–CN/C–S bond reconstruction to introduce both sulfur and cyano groups onto a single carbon center. This cyanothiolation reaction will greatly enhance the synthetic utility of carbenoid species as new entries for the construction of diverse heteroatom-containing nitriles via cyanofunctionalization of metal–carbene species.
中文翻译:

铜催化的氰基硫醇化反应并入一个硫取代的季碳中心
含硫腈由于其硫和氰基官能团的多样的生物活性而在生命科学中具有重要的研究价值。在此,已经描述了N-甲苯磺酰hydr与硫氰酸盐的铜催化的氰基硫醇化以产生带有硫取代的季碳中心原子的α-芳基硫代烷腈。该新方案涉及铜卡宾物种促进S–CN键断裂和C–CN / C–S键重构的过程,以将硫基和氰基基团引入单个碳中心。这种氰基硫醇化反应将极大地增强类胡萝卜素物质的合成效用,作为构建各种含杂原子的腈的新途径。 金属-卡宾物种的氰基官能化。
更新日期:2017-09-25
中文翻译:

铜催化的氰基硫醇化反应并入一个硫取代的季碳中心
含硫腈由于其硫和氰基官能团的多样的生物活性而在生命科学中具有重要的研究价值。在此,已经描述了N-甲苯磺酰hydr与硫氰酸盐的铜催化的氰基硫醇化以产生带有硫取代的季碳中心原子的α-芳基硫代烷腈。该新方案涉及铜卡宾物种促进S–CN键断裂和C–CN / C–S键重构的过程,以将硫基和氰基基团引入单个碳中心。这种氰基硫醇化反应将极大地增强类胡萝卜素物质的合成效用,作为构建各种含杂原子的腈的新途径。 金属-卡宾物种的氰基官能化。










































 京公网安备 11010802027423号
京公网安备 11010802027423号