当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light Photocatalytic Decarboxylation of α,β-Unsaturated Carboxylic Acids: Facile Access to Stereoselective Difluoromethylated Styrenes in Batch and Flow
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acscatal.7b03019 Xiao-Jing Wei 1 , Wout Boon 1 , Volker Hessel 1 , Timothy Noël 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acscatal.7b03019 Xiao-Jing Wei 1 , Wout Boon 1 , Volker Hessel 1 , Timothy Noël 1
Affiliation
|
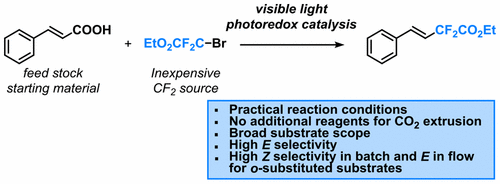
The development of synthetic methodologies which provide access to both stereoisomers of α,β-disubstituted olefins is a challenging undertaking. Herein, we describe the development of an operationally simple and stereoselective synthesis of difluoromethylated styrenes via a visible-light photocatalytic decarboxylation strategy using fac-Ir(ppy)3 as the photocatalyst. Meta- and para-substituted cinnamic acids provide the expected E-isomer. In contrast, ortho-substituted cinnamic acids yield selectively the less stable Z-product, whereas the E-isomer can be obtained via continuous-flow processing through accurate control of the reaction time. Furthermore, our protocol is amenable to the decarboxylative difluoromethylation of aryl propiolic acids.
中文翻译:

α,β-不饱和羧酸的可见光光催化脱羧:批量和流动方便地进入立体选择性二氟甲基化苯乙烯。
能够获得α,β-二取代的烯烃的两种立体异构体的合成方法的发展是一项艰巨的任务。在这里,我们描述了通过使用fac -Ir(ppy)3作为光催化剂的可见光光催化脱羧策略,开发一种操作简单且立体选择性的二氟甲基化苯乙烯合成方法。间位和对位取代的肉桂酸可提供预期的E-异构体。相反,邻位肉桂酸选择性地产生不稳定的Z产物,而E-异构体可以通过连续流处理通过精确控制反应时间来获得。此外,我们的方案适用于芳基丙酸的脱羧二氟甲基化。
更新日期:2017-09-23
中文翻译:

α,β-不饱和羧酸的可见光光催化脱羧:批量和流动方便地进入立体选择性二氟甲基化苯乙烯。
能够获得α,β-二取代的烯烃的两种立体异构体的合成方法的发展是一项艰巨的任务。在这里,我们描述了通过使用fac -Ir(ppy)3作为光催化剂的可见光光催化脱羧策略,开发一种操作简单且立体选择性的二氟甲基化苯乙烯合成方法。间位和对位取代的肉桂酸可提供预期的E-异构体。相反,邻位肉桂酸选择性地产生不稳定的Z产物,而E-异构体可以通过连续流处理通过精确控制反应时间来获得。此外,我们的方案适用于芳基丙酸的脱羧二氟甲基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号