Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ion Transport in Confined Geometries below the Nanoscale: Access Resistance Dominates Protein Channel Conductance in Diluted Solutions
ACS Nano ( IF 15.8 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acsnano.7b05529 Antonio Alcaraz 1 , M. Lidón López 1 , María Queralt-Martín 1 , Vicente M. Aguilella 1
ACS Nano ( IF 15.8 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acsnano.7b05529 Antonio Alcaraz 1 , M. Lidón López 1 , María Queralt-Martín 1 , Vicente M. Aguilella 1
Affiliation
|
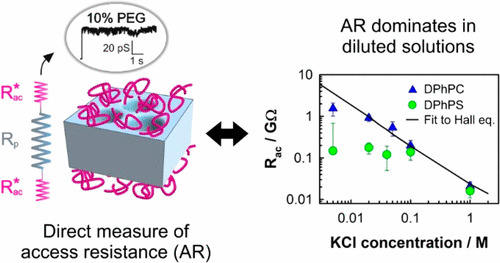
Synthetic nanopores and mesoscopic protein channels have common traits like the importance of electrostatic interactions between the permeating ions and the nanochannel. Ion transport at the nanoscale occurs under confinement conditions so that the usual assumptions made in microfluidics are challenged, among others, by interfacial effects such as access resistance (AR). Here, we show that a sound interpretation of electrophysiological measurements in terms of channel ion selective properties requires the consideration of interfacial effects, up to the point that they dominate protein channel conductance in diluted solutions. We measure AR in a large ion channel, the bacterial porin OmpF, by means of single-channel conductance measurements in electrolyte solutions containing varying concentrations of high molecular weight PEG, sterically excluded from the pore. Comparison of experiments performed in charged and neutral planar membranes shows that lipid surface charges modify the ion distribution and determine the value of AR, indicating that lipid molecules are more than passive scaffolds even in the case of large transmembrane proteins. We also found that AR may reach up to 80% of the total channel conductance in diluted solutions, where electrophysiological recordings register essentially the AR of the system and depend marginally on the pore characteristics. These findings may have implications for several low aspect ratio biological channels that perform their physiological function in a low ionic strength and macromolecule crowded environment, just the two conditions enhancing the AR contribution.
中文翻译:

纳米尺度以下的受限几何结构中的离子运输:稀释溶液中的通道阻力主导蛋白质通道电导
合成的纳米孔和介观的蛋白质通道具有共同的特征,例如渗透离子与纳米通道之间静电相互作用的重要性。纳米级的离子传输发生在封闭条件下,因此,微流体学中的通常假设受到界面效应(例如,进入阻力(AR))的挑战。在这里,我们表明,根据通道离子选择性特性对电生理学测量进行合理的解释需要考虑界面效应,直至它们在稀释溶液中主导蛋白质通道电导率为止。我们通过在包含不同浓度的高分子量PEG的电解质溶液中进行单通道电导测量来测量大离子通道(细菌性孔蛋白OmpF)中的AR,在空间上不包含在毛孔中。在带电膜和中性平面膜上进行的实验比较表明,脂质表面电荷会改变离子分布并确定AR值,这表明即使在跨膜蛋白较大的情况下,脂质分子也比被动支架更多。我们还发现,在稀释溶液中,AR可能会达到总通道电导的80%,其中电生理记录基本上记录了系统的AR,并在一定程度上取决于孔的特性。这些发现可能对在低离子强度和大分子拥挤环境中执行其生理功能的几个低长宽比的生物通道有影响,仅这两个条件增强了AR的贡献。在带电膜和中性平面膜上进行的实验比较表明,脂质表面电荷会改变离子分布并确定AR值,这表明即使在跨膜蛋白较大的情况下,脂质分子也比被动支架更多。我们还发现,在稀释溶液中,AR可能会达到总通道电导的80%,其中电生理记录基本上记录了系统的AR,并在一定程度上取决于孔的特性。这些发现可能对在低离子强度和大分子拥挤环境中执行其生理功能的几个低长宽比的生物通道有影响,仅这两个条件增强了AR的贡献。在带电膜和中性平面膜上进行的实验比较表明,脂质表面电荷会改变离子分布并确定AR值,这表明即使在跨膜蛋白较大的情况下,脂质分子也比被动支架更多。我们还发现,在稀释溶液中,AR可能会达到总通道电导的80%,其中电生理记录基本上记录了系统的AR,并在一定程度上取决于孔的特性。这些发现可能对在低离子强度和大分子拥挤环境中执行其生理功能的几个低长宽比的生物通道有影响,仅这两个条件增强了AR的贡献。
更新日期:2017-09-23
中文翻译:

纳米尺度以下的受限几何结构中的离子运输:稀释溶液中的通道阻力主导蛋白质通道电导
合成的纳米孔和介观的蛋白质通道具有共同的特征,例如渗透离子与纳米通道之间静电相互作用的重要性。纳米级的离子传输发生在封闭条件下,因此,微流体学中的通常假设受到界面效应(例如,进入阻力(AR))的挑战。在这里,我们表明,根据通道离子选择性特性对电生理学测量进行合理的解释需要考虑界面效应,直至它们在稀释溶液中主导蛋白质通道电导率为止。我们通过在包含不同浓度的高分子量PEG的电解质溶液中进行单通道电导测量来测量大离子通道(细菌性孔蛋白OmpF)中的AR,在空间上不包含在毛孔中。在带电膜和中性平面膜上进行的实验比较表明,脂质表面电荷会改变离子分布并确定AR值,这表明即使在跨膜蛋白较大的情况下,脂质分子也比被动支架更多。我们还发现,在稀释溶液中,AR可能会达到总通道电导的80%,其中电生理记录基本上记录了系统的AR,并在一定程度上取决于孔的特性。这些发现可能对在低离子强度和大分子拥挤环境中执行其生理功能的几个低长宽比的生物通道有影响,仅这两个条件增强了AR的贡献。在带电膜和中性平面膜上进行的实验比较表明,脂质表面电荷会改变离子分布并确定AR值,这表明即使在跨膜蛋白较大的情况下,脂质分子也比被动支架更多。我们还发现,在稀释溶液中,AR可能会达到总通道电导的80%,其中电生理记录基本上记录了系统的AR,并在一定程度上取决于孔的特性。这些发现可能对在低离子强度和大分子拥挤环境中执行其生理功能的几个低长宽比的生物通道有影响,仅这两个条件增强了AR的贡献。在带电膜和中性平面膜上进行的实验比较表明,脂质表面电荷会改变离子分布并确定AR值,这表明即使在跨膜蛋白较大的情况下,脂质分子也比被动支架更多。我们还发现,在稀释溶液中,AR可能会达到总通道电导的80%,其中电生理记录基本上记录了系统的AR,并在一定程度上取决于孔的特性。这些发现可能对在低离子强度和大分子拥挤环境中执行其生理功能的几个低长宽比的生物通道有影响,仅这两个条件增强了AR的贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号