PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-09-22 , DOI: 10.1371/journal.ppat.1006625 Yong-Xin Zhang , Yu-Ming Huang , Quan-Jie Li , Xiao-Yu Li , Yong-Dong Zhou , Fei Guo , Jin-Ming Zhou , Shan Cen
|
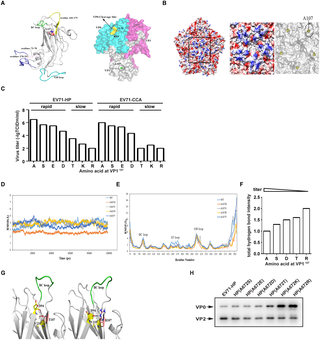
Enterovirus 71 (EV71) is the major causative agent of hand, foot and mouth disease (HFMD) in children, causing severe clinical outcomes and even death. Here, we report an important role of the highly conserved alanine residue at position 107 in the capsid protein VP1 (VP1A107) in the efficient replication of EV71. Substitutional mutations of VP1A107 significantly diminish viral growth kinetics without significant effect on viral entry, expression of viral genes and viral production. The results of mechanistic studies reveal that VP1A107 regulates the efficient cleavage of the VP0 precursor during EV71 assembly, which is required, in the next round of infection, for the transformation of the mature virion (160S) into an intermediate or A-particle (135S), a key step of virus uncoating. Furthermore, the results of molecular dynamic simulations and hydrogen-bond networks analysis of VP1A107 suggest that flexibility of the VP1 BC loop or the region surrounding the VP1107 residue directly correlates with viral infectivity. It is possible that sufficient flexibility of the region surrounding the VP1107 residue favors VP0 conformational change that is required for the efficient cleavage of VP0 as well as subsequent viral uncoating and viral replication. Taken together, our data reveal the structural role of the highly conserved VP1A107 in regulating EV71 maturation. Characterization of this novel determinant of EV71 virulence would promote the study on pathogenesis of Enteroviruses.
中文翻译:

VP1中高度保守的氨基酸调节肠病毒71的成熟
肠病毒71(EV71)是儿童手足口病(HFMD)的主要病原体,可导致严重的临床后果甚至死亡。在这里,我们报告了衣壳蛋白VP1(VP1 A107)中位置107的高度保守的丙氨酸残基在EV71的有效复制中的重要作用。VP1 A107的取代突变大大降低了病毒的生长动力学,而对病毒的进入,病毒基因的表达和病毒产生没有显着影响。机理研究的结果表明,VP1 A107调节EV71装配过程中VP0前体的有效裂解,这在下一轮感染中是成熟病毒体(160S)转变为中间颗粒或A颗粒(135S)所需的,这是病毒脱壳的关键步骤。此外,VP1 A107的分子动力学模拟和氢键网络分析结果表明,VP1 BC环或VP1 107残基周围区域的柔韧性与病毒感染性直接相关。VP1 107周围的区域可能具有足够的灵活性残基有利于VP0的构象改变,这是有效裂解VP0以及随后的病毒脱壳和病毒复制所必需的。综上所述,我们的数据揭示了高度保守的VP1 A107在调节EV71成熟中的结构作用。EV71毒力这一新决定因素的表征将促进肠道病毒发病机理的研究。










































 京公网安备 11010802027423号
京公网安备 11010802027423号